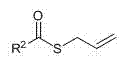
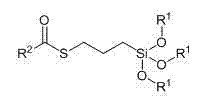
Technology for preparing terminated-type sulfur-containing silane coupling agent through hydrosilation method
A technology of sulfur silane coupling agent and hydrosilicon addition, which is applied in chemical instruments and methods, compounds of group 4/14 elements of the periodic table, organic chemistry, etc., can solve the problems affecting storage stability, alkoxysilane Unstable water, prone to hydrolysis and other problems, to achieve the effect of improving raw material utilization, improving storage stability, and inhibiting hydrolysis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] 1) Preparation of allyl thiooctanoate (C 7 h 15 COSCH 2 -CH=CH 2 )
[0025] In a 2L three-neck flask protected by nitrogen, add 240g (1mol) of sodium sulfide nonahydrate solid, add 150g of water, and dissolve to obtain a sodium sulfide solution with a concentration of 20%. Under mechanical stirring, 162.5g (1mol) of octanoyl chloride is added from The constant pressure dropping funnel was added dropwise, and the dropwise addition was completed in about 30 minutes. With the addition of octanoyl chloride, the temperature in the bottle rose to about 70°C. The system was naturally cooled to room temperature, and after stirring for 4 hours, a slightly viscous mixture was obtained. Thick aqueous solution of sodium lipoate. Add 3g of saturated methyltrioctylammonium chloride solution to the above solution, add 80.3g (1.05mol) of 3-chloropropene into the bottle under mechanical stirring, after completion, raise the temperature and keep it at a slight reflux for 5 hours, and...
Embodiment 2
[0029] 1) Preparation of allyl thiohexanoate (C 7 h 15 COSCH 2 -CH=CH 2 )
[0030] In a 2L three-necked flask protected by nitrogen gas, 622g of 18% sodium hydrosulfide aqueous solution (containing 102g of sodium hydrogensulfide, 2mol), under mechanical stirring, 134.5g (1mol) of hexanoyl chloride was dropped from a constant pressure dropping funnel to produce The hydrogen sulfide gas was absorbed with sodium hydroxide solution, and the dropwise addition was completed in about 30 minutes. After stirring at 60° C. for 3 hours, it was cooled to room temperature to obtain a slightly viscous aqueous solution of sodium thiohexanoate. Add 0.5 g of tetrabutylammonium bromide to the above solution, add 80.3 g (1.05 mol) of 3-chloropropene into the bottle under mechanical stirring, control the heating temperature, keep a slight reflux for 5 hours, and detect the organic phase by gas chromatography. - The chloropropene content is less than 3%, stop stirring, separate the upper organ...
Embodiment 3
[0034] 1) Preparation of allyl thiolaurate (C 11 h 23 COSCH 2 -CH=CH 2 )
[0035] In a 2L three-neck flask protected by nitrogen, add 130g (1mol) of sodium sulfide solid with a sodium sulfide content of 60%, add 520g of water, and dissolve to obtain a sodium sulfide solution with a concentration of 12%. Under mechanical stirring, mix 218.5g (1mol) ) Lauroyl chloride was added dropwise from a constant pressure dropping funnel, and the dropwise addition was completed in about 40 minutes, stirred at 75°C for 3 hours, cooled to room temperature, and a slightly viscous aqueous solution of sodium thiolaurate was obtained. Add 2g of trimethyldodecyl ammonium chloride to the above solution, and add 80.3g (1.05mol) of 3-chloropropene into the bottle under mechanical stirring. After completion, raise the temperature and maintain a slight reflux for 5 hours. The 3-chloropropene content is less than 3%, stop stirring, separate the upper organic layer, remove the low boiling point unde...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com