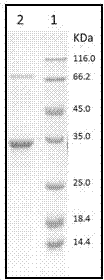
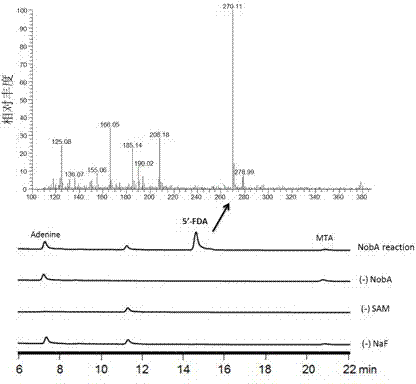
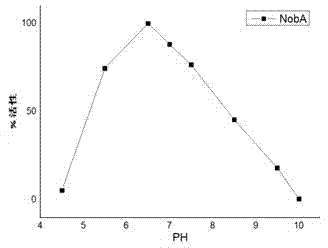
Halogenase for catalyzing formation of C-F and C-Cl bonds
A halogenase, -FDA technology, applied in the field of halogenase, can solve the problems of unsatisfactory selectivity, poor solubility of fluorine-containing inorganic substances, high toxicity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1. Determination of the Halogenase Gene Sequence
[0046] 1.1 Obtaining the original gene of halogenase
[0047] Using FramePlot 4.0beta (http: / / nocardia.nih.go.jp / fp4 / ) and NCBI database BLAST method (http: / / www.ncbi.nlm.nih.gov / blast / ) to obtain Nocardia from Brazil The original gene sequence information of the halogenase (SEQ ID No 1).
[0048] 1.2 Acquisition of Halogenase Escherichia coli Optimized Expression Sequence
[0049] The halogenase gene derived from Nocardia brasiliensis in GenScript Biotechnology Co., Ltd., using Optimum Gene TM Algorithm was used to synthesize the optimized expression gene of Escherichia coli, and the halogenase gene sequence (SEQ ID No 2) with 82% identity to the original sequence was obtained. And constructed on the pUC57 simple plasmid.
[0050]
Embodiment 2
[0051] Example 2. Construction of Halogenase Expression Plasmid
[0052] restriction endonuclease Nde I and Hin dⅢ Plasmid with Halogenase Gene Linked nob A / pUC57 simple and plasmid pET28a(+) were digested with double enzymes, and the 900bp halogenase DNA fragment and the pET28a(+) vector large fragment were recovered by electrophoresis, and then the vector and the fragment were ligated with T4 polymerase solution I from TaKaRa Company, and reacted overnight at 16°C . Then transform Escherichia coli DH5 α Competent cells were spread on LB selective plates added with kanamycin antibiotic, and incubated upside down at 37°C for 15 h. The transformant that can grow on the LB plate with kanamycin may be the transformant transformed with the recombinant plasmid, select a single colony and inoculate it in the liquid LB medium containing kanamycin, and culture it on a shaker at 37 °C and 220 rpm for 15 h , to amplify the pET28a(+) plasmid that may contain the halogenase gene. ...
Embodiment 3
[0054] Embodiment 3. The acquisition of engineering bacteria
[0055] Transform the correct recombinant plasmid into Escherichia coli BL21 (DE 3 ) Competent cells, the genetically engineered bacteria pWHU2401 / BL21 (DE 3 ), and finally spread the obtained engineered bacteria on the LB selective plate added with kanamycin antibiotic, culture it upside down at 37 ℃ for about 20 h, and grow on the LB plate added with kanamycin antibiotic It is the transformant transformed with the recombinant plasmid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com