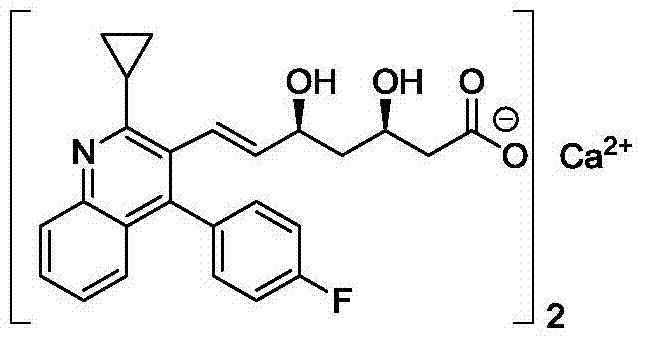
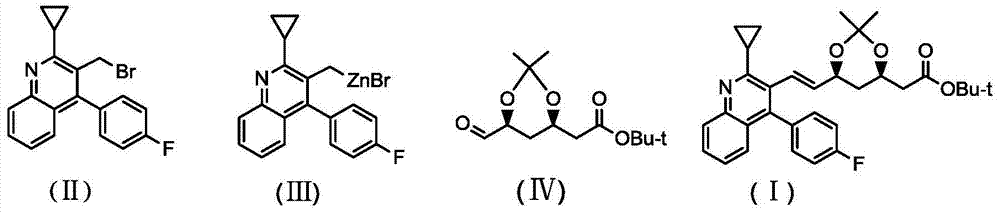
New method of pitavastatin calcium key intermediate
A technology for pitavastatin calcium and intermediates, which is applied in the field of preparation of key intermediates of pitavastatin calcium by highly active organic zinc reagents, and can solve the problems of low yield, difficult separation and purification, and many Z-isomer residues. Achieve the effect of high configuration purity, simple purity, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Preparation of lithium naphthalene reagent:
[0030] Under the protection of nitrogen, metal lithium (0.4 g, 60 mmol) was added to anhydrous naphthalene (2.6 g, 20 mmol), and stirred at 20-25° C. for 2 h to obtain lithium naphthalene reagent.
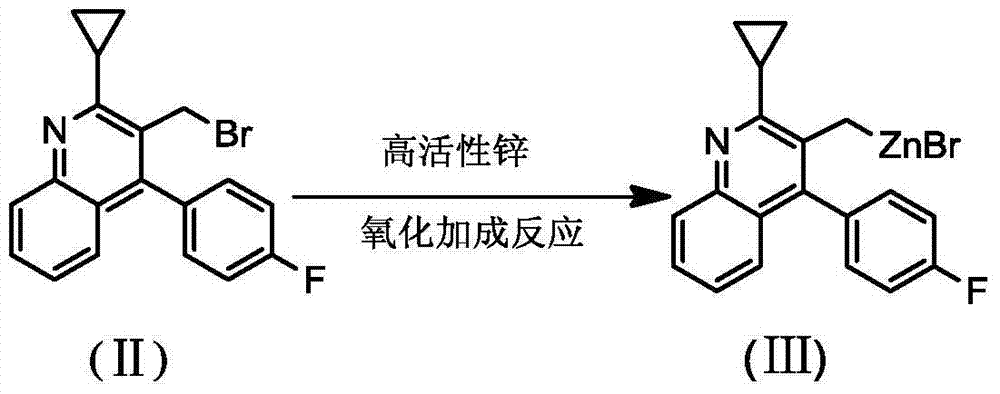
[0031] The preparation of organic zinc reagent (Ⅲ), reaction formula is as follows:
[0032]
[0033] Operation steps: under the protection of nitrogen, add compound (II) (10.7g, 30mmol) and 50ml of anhydrous tetrahydrofuran to a 250ml reaction flask, stir and cool to 10-20°C, add zinc bromide (13.5g, 60mmol) in batches , stirred for 10 minutes, added dropwise the above naphthalenelithium reagent, after the reaction temperature was stabilized, heated to reflux (65-75°C), and stirred for 5-6h. The resulting reaction solution is the organozinc reagent (Ⅲ).
[0034] The preparation of compound (I), reaction formula is as follows:
[0035]
[0036] Operation steps: add K to the organozinc reagent (Ⅲ) 2 CO 3 (12.4g, 90mmol)...
Embodiment 2
[0040] The preparation of organic zinc reagent (Ⅲ), reaction formula is as follows:
[0041]
[0042] Operation steps: under the protection of nitrogen, add compound (II) (10.7g, 30mmol) and 50ml of anhydrous tetrahydrofuran into a 250ml reaction flask, stir and cool to 10-20°C, add zinc bromide (13.5g, 60mmol), stir For 10 minutes, lithium metal (0.4 g, 60 mmol) was added in batches, and after the reaction temperature stabilized, it was heated to reflux (65-75° C.), and stirred for 5-6 hours. The resulting reaction solution is the organozinc reagent (Ⅲ).
[0043] The preparation of compound (I), reaction formula is as follows:
[0044]
[0045] Operation steps: Add potassium carbonate (12.4g, 90mmol) to organozinc reagent (Ⅲ), stir at 0-10°C under temperature control; dissolve compound (Ⅳ) (7.8g, 30mmol) in tetrahydrofuran (15ml) and stir to dissolve and clarify , drop into the above-mentioned organozinc reagent (Ⅲ), about 0.5h drop. The temperature was raised to 25-...
Embodiment 3
[0049] Preparation of lithium naphthalene reagent:
[0050] Under the protection of nitrogen, metal lithium (0.4 g, 60 mmol) was added to anhydrous naphthalene (2.6 g, 20 mmol), and stirred at 20-25° C. for 2 h to obtain lithium naphthalene reagent.
[0051] The preparation of organic zinc reagent (Ⅲ), reaction formula is as follows:
[0052]
[0053] Operation steps: under the protection of nitrogen, add compound (II) (10.7g, 30mmol) and 50ml of anhydrous tetrahydrofuran into a 250ml reaction flask, stir and cool to 10-20°C, add zinc bromide (13.5g, 60mmol) in batches , stirred for 10 minutes, added dropwise the above naphthalenelithium reagent, after the reaction temperature was stabilized, heated to reflux (65-75°C), and stirred for 5-6h. The resulting reaction solution is the organozinc reagent (Ⅲ).
[0054] The preparation of compound (I), reaction formula is as follows:
[0055]
[0056] Operation steps: add K to the organozinc reagent (Ⅲ) 2 CO 3 (12.4g, 90mmo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com