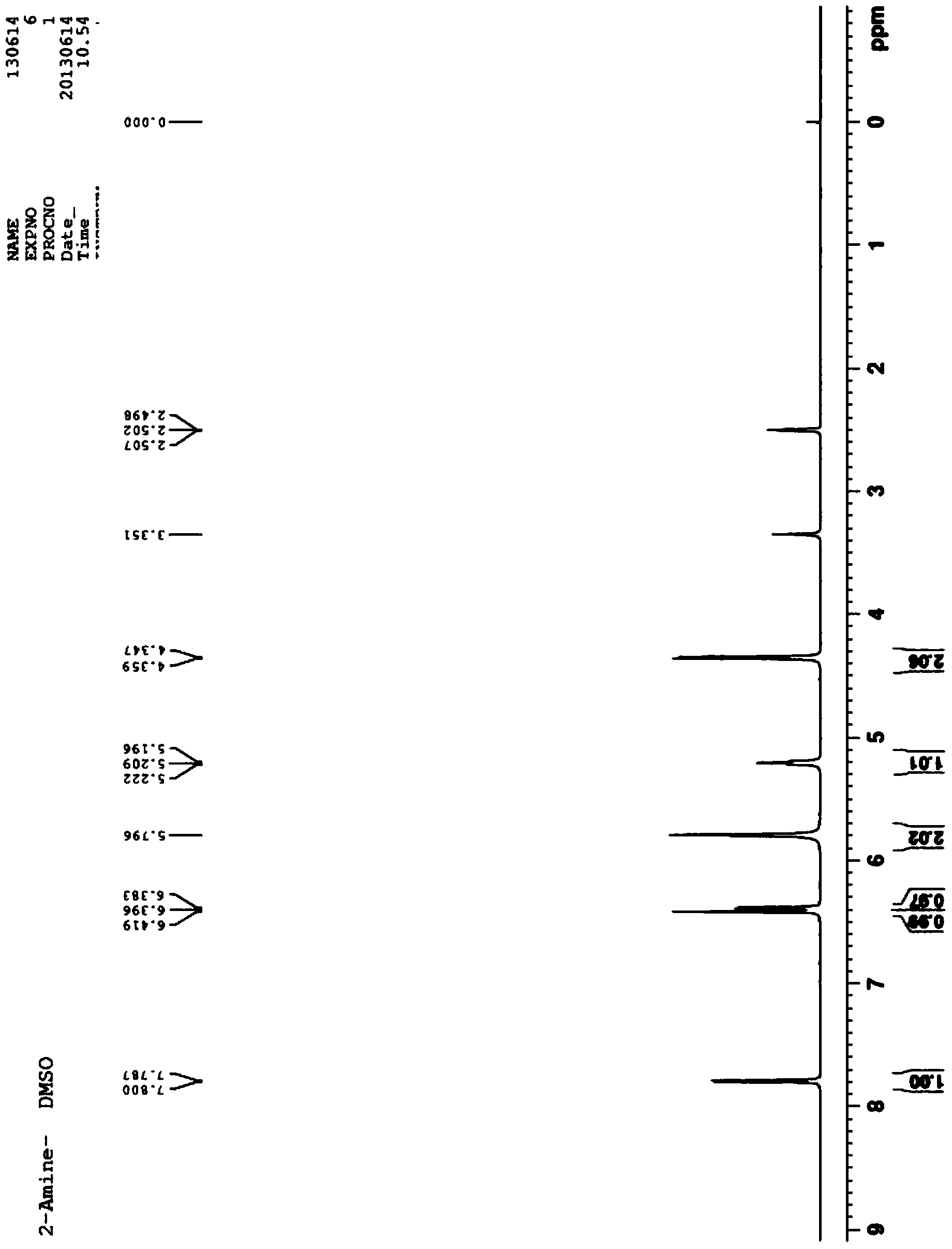
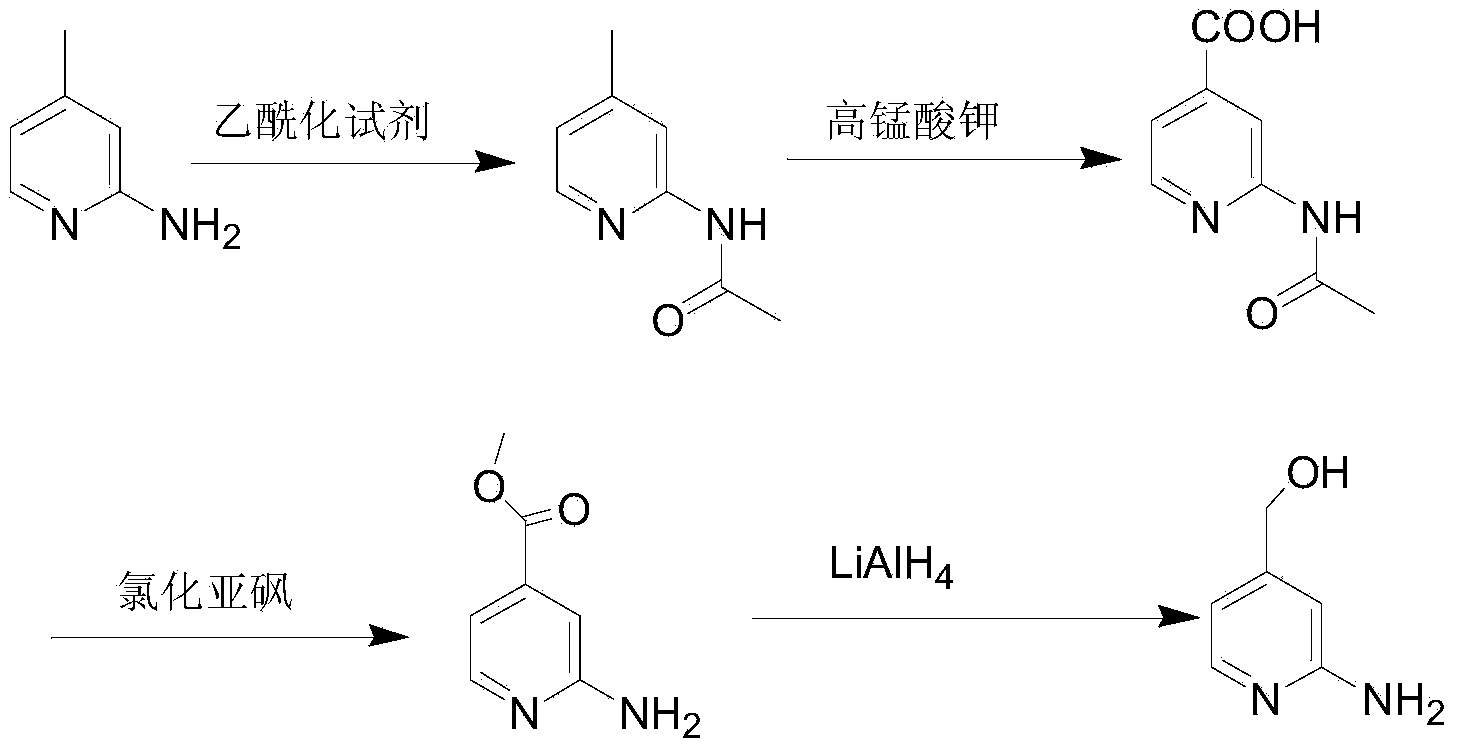
Preparation method of 2-aminopyridine-4-methyl alcohol
A technology of aminopyridine and methanol, which is applied in the field of preparation of 2-aminopyridine-4-methanol, can solve the problems of difficult industrial production, low process yield, serious environmental pollution, etc., to avoid the generation of waste liquid and waste residue, shorten the Process flow and the effect of reducing production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] (1) Preparation of methyl 2-chloroisonicotinate
[0026] Add methanol (316mL, 250g) and 2-chloroisonicotinic acid (50.0g, 0.32mol) into the four-neck flask, cool down to 0°C, add thionyl chloride (114.2g, 0.96mol) dropwise, and control the temperature at 0°C After the dropwise addition, raise the temperature to room temperature and stir for 30 minutes, raise the temperature and reflux for 4 hours, and conduct thin-layer chromatography analysis. After the reaction is completed, methanol is distilled off, dissolved in 200 mL of water, and sodium carbonate aqueous solution (10% by mass) is added dropwise to adjust the pH=8- 9. Extract with 300mL×2 ethyl acetate, wash the organic phase once with water, dry sodium sulfate, and concentrate to obtain colorless liquid methyl 2-chloroisonicotinate (51.3g, yield 93.5%).
[0027] (2) Preparation of 2-chloropyridine-4-methanol
[0028] Add tetrahydrofuran (289mL, 257.0g), methyl 2-chloroisonicotinate (51.4g, 0.30mol) and lithium c...
Embodiment 2
[0032] (1) Preparation of ethyl 2-chloroisonicotinate
[0033] Add ethanol (643mL, 500.0g), 2-chloroisonicotinic acid (50.0g, 0.32mol) into the four-neck flask, cool down to 3°C, add thionyl chloride (38.1g, 0.32mol) dropwise, and control the temperature for 3 ℃, after the dropwise addition, raise the temperature to room temperature and stir for 30min, raise the temperature and reflux for 5h, analyze by thin-layer chromatography, after the reaction is completed, distill off ethanol, add 200mL water to dissolve, add dropwise sodium carbonate aqueous solution (10% by mass) to adjust pH=8 -9, extracted with ethyl acetate 300mL×2, the organic phase was washed once with water, dried and concentrated to obtain colorless liquid ethyl 2-chloroisonicotinate (53.8g, yield 90.6%)
[0034] (2) Preparation of 2-chloropyridine-4-methanol
[0035] Add ethanol (606mL, 478.4g), ethyl 2-chloroisonicotinate (55.6g, 0.30mol), zinc chloride (26.6g, 0.20mol) to a four-neck flask, cool down to 5°C,...
Embodiment 3
[0039] (1) Preparation of isopropyl 2-chloroisonicotinate
[0040] Add isopropanol (954mL, 750.0g), 2-chloroisonicotinic acid (50.0g, 0.32mol) into the four-neck flask, cool down to 5°C, add thionyl chloride (57.1g, 0.48mol) dropwise, control The temperature is 5°C, the dropwise addition is completed, raised to room temperature and stirred for 30 minutes, heated to reflux for 6 hours, analyzed by thin-layer chromatography, after the reaction is completed, evaporate isopropanol, add 200mL of water to dissolve, add dropwise sodium carbonate aqueous solution (10% by mass) Adjust pH=8-9, extract with ethyl acetate 300mL×2, wash the organic phase once with water, dry sodium sulfate, concentrate to obtain colorless liquid isopropyl 2-chloroisonicotinate (62.6g, yield 98.0%)
[0041] (2) Preparation of 2-chloropyridine-4-methanol
[0042] Add methanol (211mL, 166.8g), isopropyl 2-chloroisonicotinate (59.8g, 0.30mol) and aluminum chloride (68g, 0.51mol) to a four-neck flask, cool dow...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com