Liquid chromatographic analysis method for determining content of oxolinic acid
A liquid chromatography analysis, oxolinic acid technology, applied in the direction of measuring devices, analysis materials, material separation, etc., can solve the problems of small measurement range, high measurement results, unsatisfactory, etc., to achieve simple measurement methods, high sensitivity, exclusive good sex effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
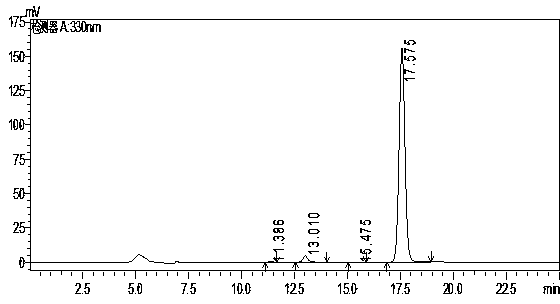
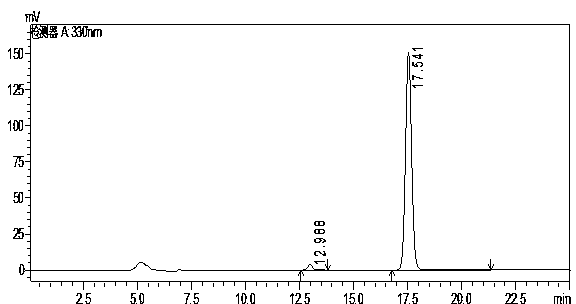
[0026] A chromatographic column using octadecyl bonded silica gel as the stationary phase, column length 150mm x inner diameter 4.6mm x packing particle size 5μm, mobile phase is acetonitrile: tetrahydrofuran (containing 0.03% tri-n-octylamine in the total volume of the mobile phase): Water (containing 0.3% citric acid in the total volume of the mobile phase) = 20:10:70, the flow rate is 0.5mL / min, the detection wavelength is 330nm, the column temperature is 40°C, and the injection volume is 10μL. Accurately weigh 20mg of oxolinic acid sample (batch number 130801) and reference substance respectively, add appropriate amount of mixed solvent of dimethylformamide and methanol (volume ratio: 2:3) to a 100mL volumetric flask, and dissolve in a water bath at 80°C After that, add the above-mentioned mixed solvent of dimethylformamide and methanol to dilute to the mark, and filter with a 0.45 μm microporous membrane before sample injection for chromatographic analysis.
[0027] Get a...
Embodiment 2
[0029] A chromatographic column using octadecyl bonded silica gel as the stationary phase, column length 150mm x inner diameter 4.6mm x packing particle size 10μm, mobile phase is acetonitrile: tetrahydrofuran (containing 0.04% tri-n-octylamine in the total volume of the mobile phase): Water (containing 0.3% citric acid in the total volume of the mobile phase) = 20:10:70, the flow rate is 0.45mL / min, the detection wavelength is 320nm, the column temperature is 35°C, and the injection volume is 10μL. Accurately weigh 20mg of oxolinic acid sample (batch number 130802) and the reference substance respectively, put into a 100mL volumetric flask, add an appropriate amount of mixed solvent of dimethylformamide and methanol (volume ratio: 2:3), and dissolve in a 78°C water bath After that, add the above-mentioned mixed solvent of dimethylformamide and methanol to dilute to the mark, and filter with a 0.45 μm microporous membrane before sample injection for chromatographic analysis.
...
Embodiment 3
[0032] A chromatographic column using octadecyl bonded silica gel as the stationary phase, column length 150mm x inner diameter 4.6mm x packing particle size 5μm, mobile phase is acetonitrile: tetrahydrofuran (containing 0.03% tri-n-octylamine in the total volume of the mobile phase): Water (containing 0.4% citric acid in the total volume of the mobile phase) = 20:10:70, the flow rate is 0.55mL / min, the detection wavelength is 310nm, the column temperature is 40°C, and the injection volume is 20μL. Accurately weigh 20 mg of oxolinic acid sample (batch number 130803) and reference substance respectively, put into a 100 mL volumetric flask, add an appropriate amount of mixed solvent of dimethylformamide and methanol (volume ratio: 2:3), and dissolve in a water bath at 80 °C After that, add the above-mentioned mixed solvent of dimethylformamide and methanol to dilute to the mark, and filter with a 0.22 μm microporous membrane before sample injection for chromatographic analysis.
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com