Method for producing alpha-phenylpyruvic acid efficiently through whole cell transformation
A technology of whole cell conversion and phenylpyruvate, applied in the field of fermentation engineering, can solve the problems of pollution, low yield, high cost, etc., and achieve the effect of solving high energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Embodiment 1L-Amino acid deaminase recombinant plasmid construction
[0021] Using P. mirabilis KCTC2566 genomic DNA as a template, use the following primers to amplify the target gene:
[0022] 5'CGC GGATCC ATGAACATTTCAAGGAGAAAGCTAC3' (forward primer) and
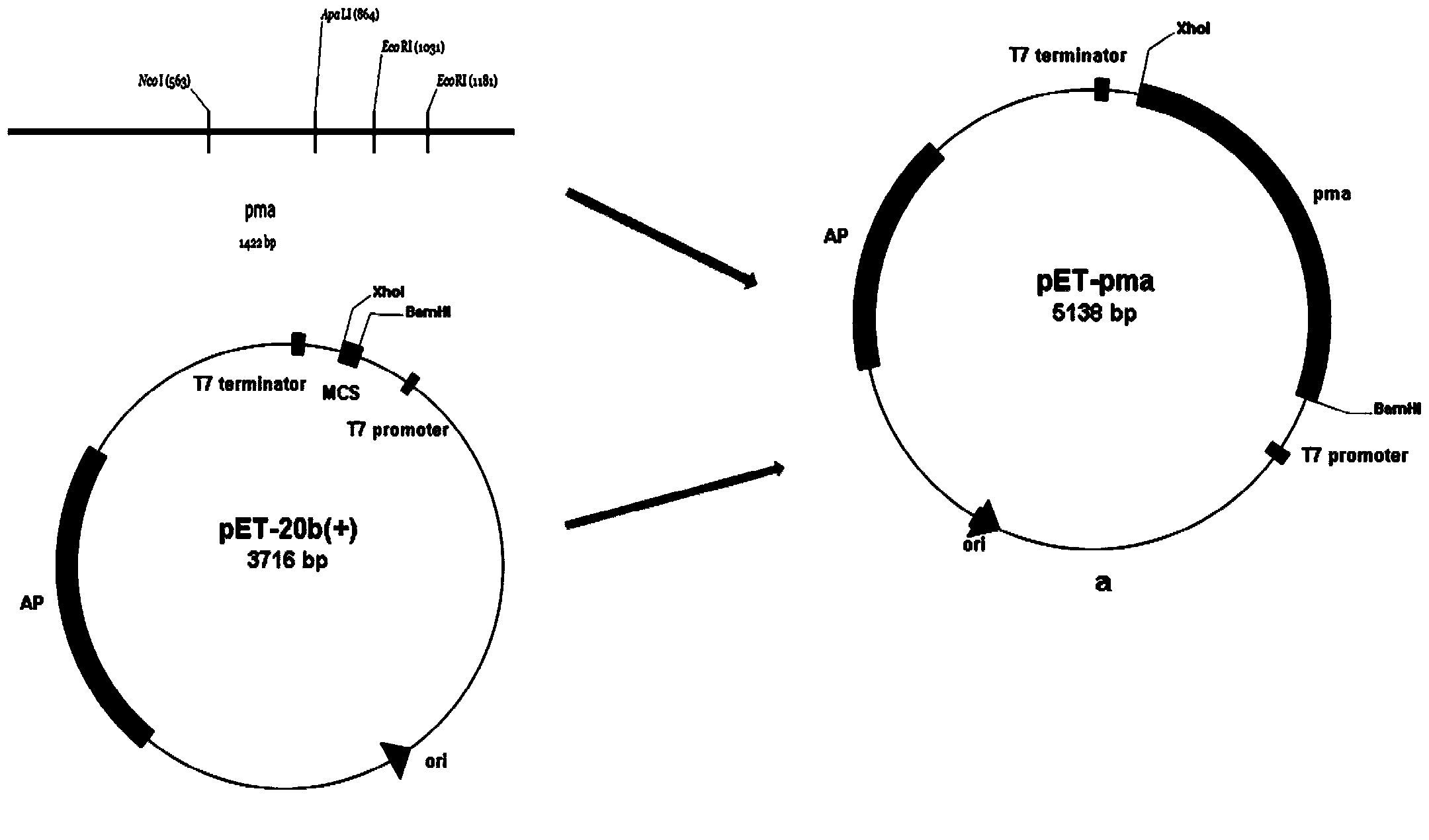
[0023] 5'CCG CTCGAG TTACTTCTTAAAACGATCCAAACTAA3' (reverse primer), the underlined parts are the restriction sites of BamH I and Xho I, respectively. The obtained target gene sequence is shown in SEQ ID NO.1. After the PCR product is purified and digested, it is connected with the vector pET-20b(+) to construct the recombinant plasmid pET-pma (such as figure 1 ).
Embodiment 2
[0024] Embodiment 2 recombinant escherichia coli construction
[0025] Transform the recombinant plasmid pET-pma of Example 1 into the cloning host E.coli JM109, pick a single colony grown on the LB ampicillin resistance plate, PCR amplify, pick out the positive transformant to extract the plasmid, double enzyme digestion verification, the band Sequencing is performed correctly, and the expression host E.coli BL21 is transformed and expressed with the correct sequence. Transformed BL21 plate single colony inoculation seed medium for overnight culture, inoculated 1% of the inoculum into the fermentation medium, cultured at 37°C until OD was 1.0, added IPTG with a final concentration of 0.4mM, and induced at 30°C for 5h.
Embodiment 3
[0026] Example 3 Whole Cell Conversion L-Phenylalanine Condition Optimization
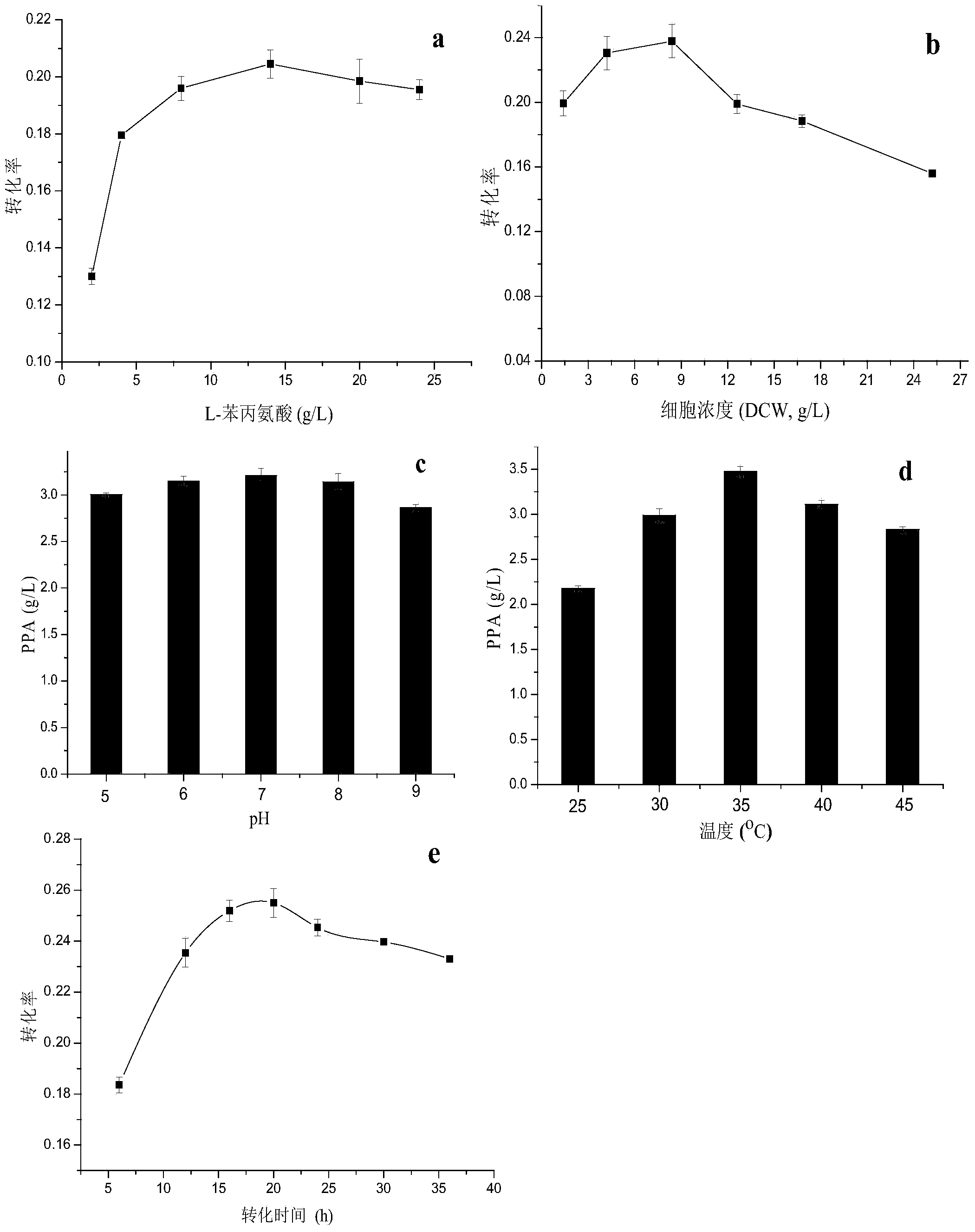
[0027] The recombinant Escherichia coli-induced cells of Example 2 were collected, and transformed into whole cells in a Tris-HCl (pH=7.6) solution. When the cell concentration is 12.6g / L (dry cell weight), in different concentrations of L-phenylalanine (2g / L, 4g / L, 8g / L, 14g / L, 20g / L, 24g / L) conditions at 37°C for 12 hours to investigate the effect of the substrate concentration on the transformation. When the optimal L-phenylalanine concentration was 14g / L, the conversion rate reached a maximum of 20.4%; when the L-phenylalanine concentration was 14g / L , adding different concentrations of biocatalysts (1.4g / L, 4.2g / L, 8.4g / L, 12.6g / L, 16.8g / L, 25.2g / L, cell dry weight), 37 ℃, transformation 12h, research The effect of cell concentration on transformation, when the optimal cell concentration is 8.4g / L, the conversion rate is 23.7%; under the condition of optimal substrate concentration and cell c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com