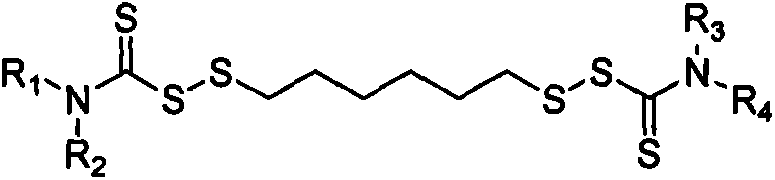
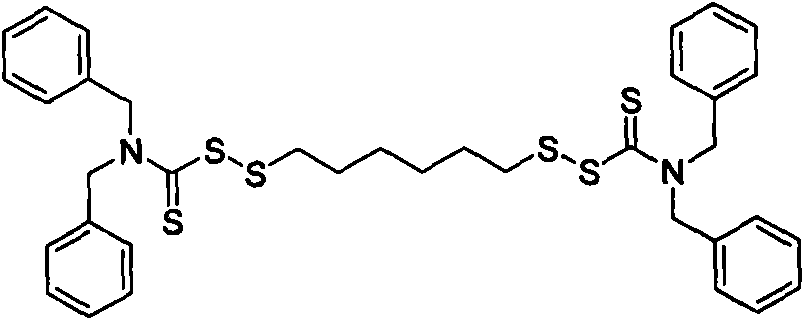
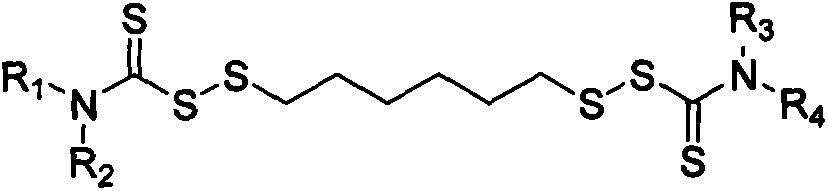
Thiocarbamic acid derivative and preparation method thereof
A technology of thiocarbamic acid and derivatives, applied in organic chemistry and other fields, can solve problems such as easy contact with air, reduce safety, and pollute the environment, and achieve the effects of simple operation, reduced energy consumption, and lowered reaction temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The first reaction process: use water as solvent.
[0033]Using a 250mL four-neck flask equipped with a stirring device, a thermometer and a reflux condenser, weigh 14.2g of hexamethylene dihydrate 1,6-dithiosulfate disodium salt (Duralink HTS, purchased from Flexsys) and add it to the flask , add 36.4g of water, stir to dissolve HTS, add 3.2g of sodium bicarbonate, 3.4g of formaldehyde solution with a concentration of 35% by mass, stir at 20°C for 30min, then add 9.2g of dichloromethane. Then 94.5 g of a sodium dibenzyl dithiocarbamate solution (sodium dibenzyl dithiocarbamate, purchased from Bailingwei Technology Co., Ltd., purity: 98%) with a mass percentage concentration of 25% was added dropwise, and the reaction was stirred at 20° C. for 5 h . Suction filter the above solution, wash the obtained solid product with water, and dry it in an oven at 60°C to constant weight to obtain 26.4g of 1,6-bis(N,N-dibenzylthiocarbamoyl disulfide)hexane solid powder , The calcu...
Embodiment 2
[0037] The first reaction process: use water as solvent.
[0038] Using a 250mL four-neck flask equipped with a stirring device, a thermometer and a reflux condenser, weigh 8.2g of HTS and add it to the flask, add 80g of water, stir to dissolve the HTS, add 1.0g of sodium bicarbonate, 3.5g of mass percent 20% formaldehyde aqueous solution, stirred at 20°C for 30min, and added 5.9g of dichloromethane. Then 60.0 g of 20% by mass concentration of sodium dibenzyl dithiocarbamate solution was added dropwise, and stirred at 20° C. for 5 h. Suction filter the above solution, wash the obtained solid product with water, and dry it in an oven at 60°C to constant weight to obtain 6.6g of 1,6-bis(N,N-dibenzylthiocarbamoyl disulfide)hexane solid powder , The calculated product yield is about 92.7%.
[0039] The second reaction process: using the filtrate after suction filtration as a solvent to continuously prepare 1,6-bis(N,N-dibenzylthiocarbamoyl disulfide)hexane.
[0040] Weigh 80g o...
Embodiment 3
[0042] The first reaction process: use water as solvent.
[0043] Using a 250mL four-necked flask equipped with a stirring device, a thermometer and a reflux condenser, weigh 14.5g of HTS and add it to the flask, add 24g of water, stir to dissolve the HTS, add 5.2g of sodium bicarbonate, 9.2g of mass percent concentration 20% aqueous formaldehyde solution, stirred at 25°C for 30min, and added 1.7g of dichloromethane. Then 102.0 g of sodium dibenzyl dithiocarbamate solution with a concentration of 25% by mass was added dropwise, and stirred at 25° C. for 4 h. Suction filter the above solution, wash the obtained solid product with water, and dry it in an oven at 70°C to constant weight to obtain 13.6g of 1,6-bis(N,N-dibenzylthiocarbamoyl disulfide)hexane solid powder , The calculated product yield is about 95.4%.
[0044] The second reaction process: using the filtrate after suction filtration as a solvent to continuously prepare 1,6-bis(N,N-dibenzylthiocarbamoyl disulfide)hex...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com