Methods of administering beta7 integrin antagonists
A technology of integrins and antagonists, applied in the fields of anti-inflammatory agents, immunoglobulins, chemical instruments and methods, etc., can solve problems such as inconvenience of patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
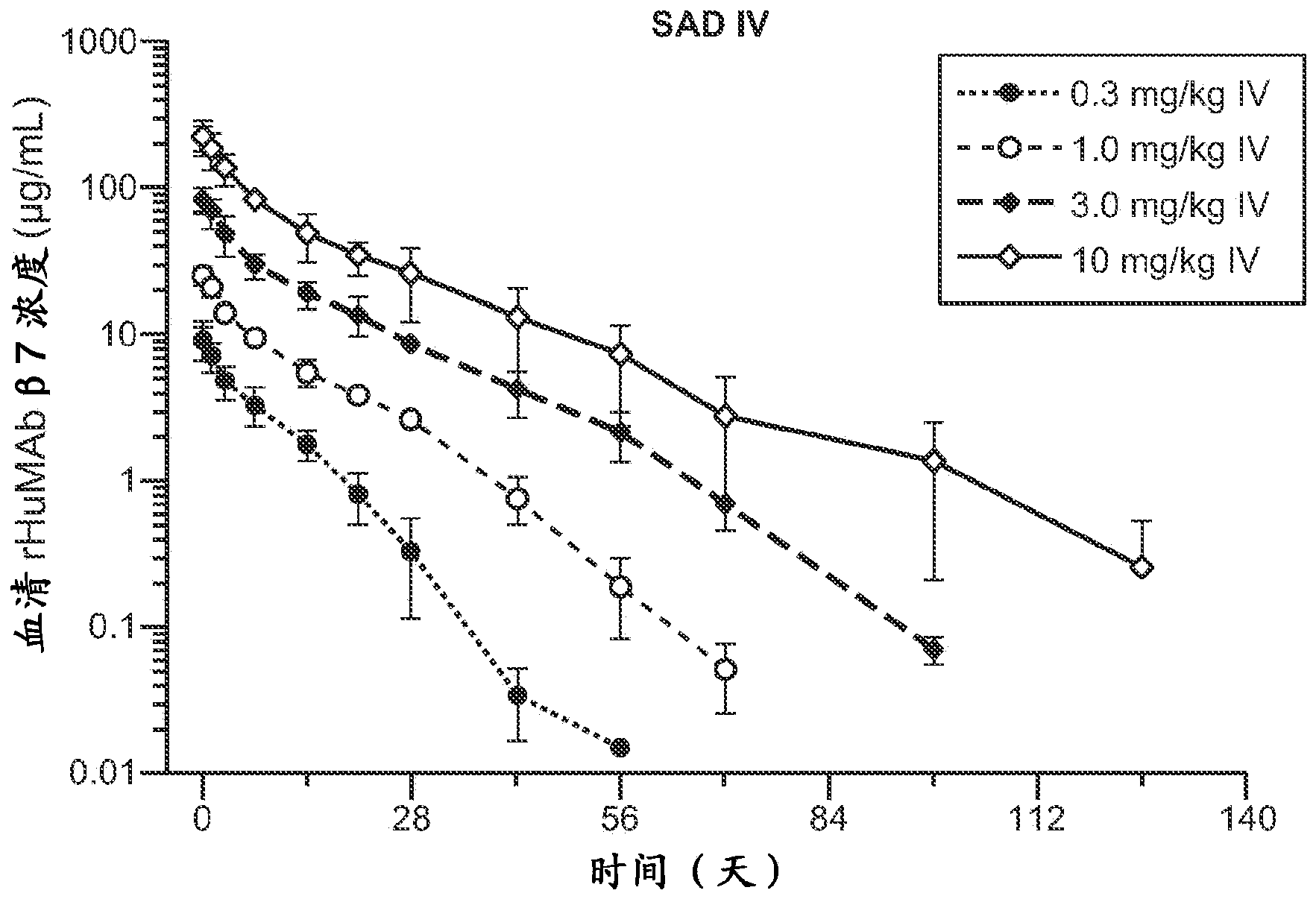
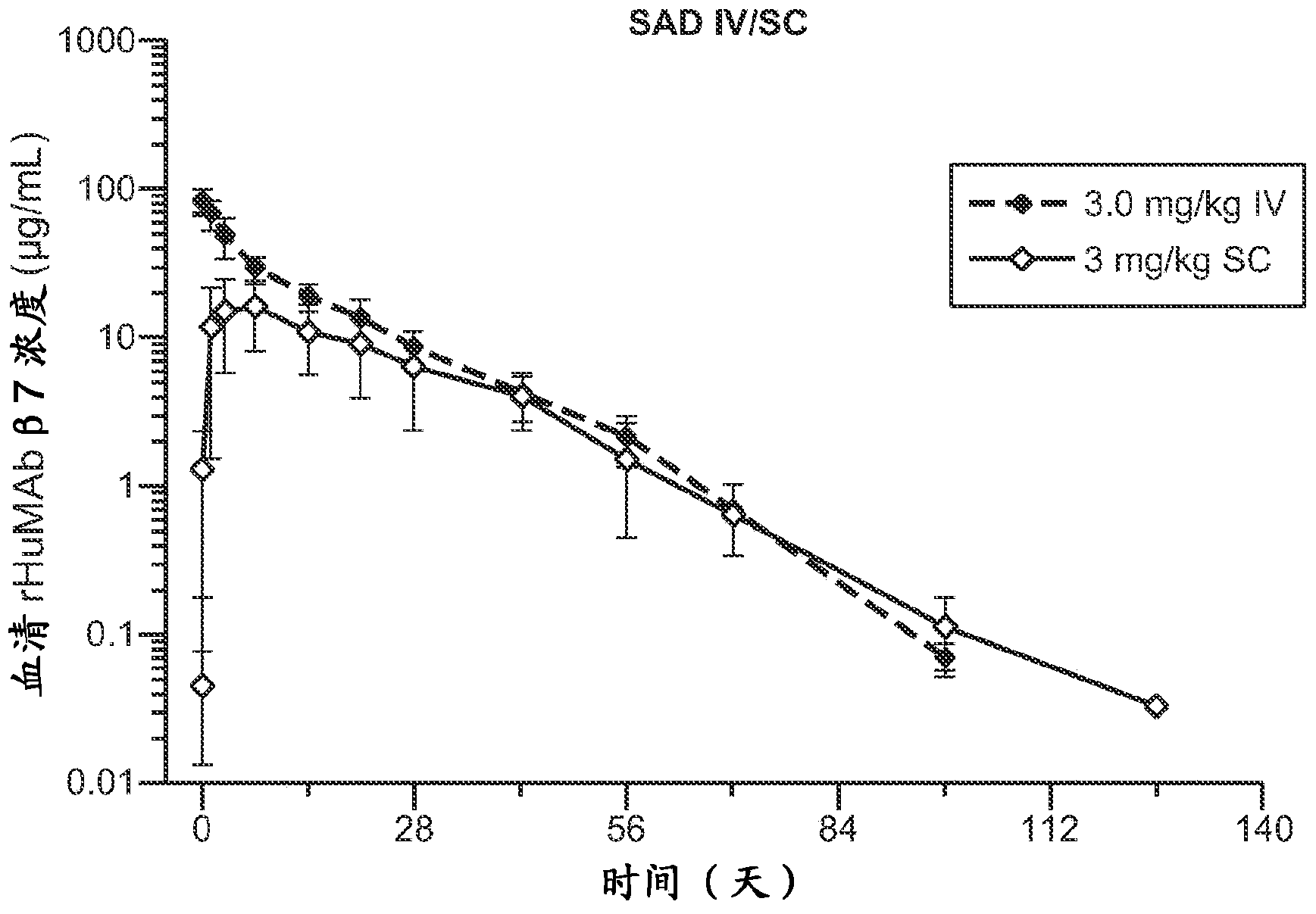
[0249] Phase I, Phase I, Evaluating the Safety, Pharmacokinetics, Pharmacodynamics, and Immunogenicity of Intravenous and Subcutaneous rhuMAbβ7 Administered in a Single-Dose, Dose-Escalation Phase Followed by a Multiple-Dose, Parallel Treatment Phase in Ulcerative Colitis Patients Multicenter, randomized, placebo-controlled, double-blind study
[0250] research description
[0251] Description of rhuMAbβ7 (etrolizumab)
[0252] rhuMAbβ7 (etrolizumab) is a humanized monoclonal antibody based on human IgG1 subclass III V H , κ subtype-IV L Consensus sequence, and specificity against the β7 subunit of integrin heterodimers. It has been shown to bind α4β7 with high affinity (K of about 116 pM d ) and αEβ7 (K about 1800pM d ).
[0253] This recombinant antibody has two heavy chains (446 residues) and two light chains (214 residues) covalently linked by interchain and intrachain disulfide bonds typical of IgG1 antibodies. For the studies described here, it was produced in Chi...
Embodiment 2
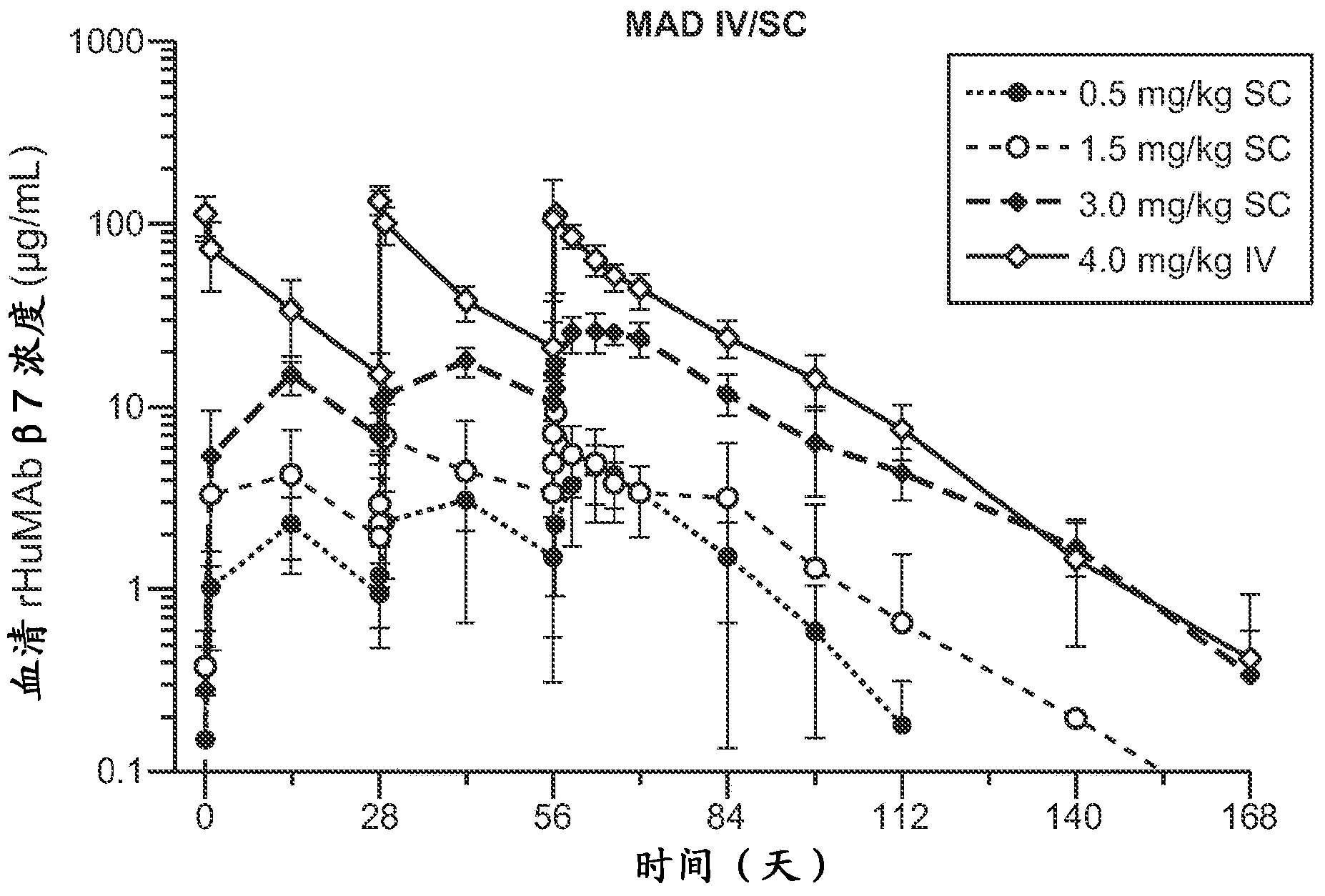
[0347] A phase II, randomized, double-blind, placebo-controlled study and an open-label extension study evaluating the efficacy and safety of rhumab beta7 in patients with moderate to severe ulcerative colitis
[0348] Research design
[0349] research description
[0350] This Phase II study is a randomized, double-blind, placebo-controlled multicenter study evaluating the efficacy and safety of two rhumab beta7 dose levels compared to placebo in patients with moderate to severe UC. This target patient population is the same as that studied in the Phase I study above. The primary efficacy endpoint will be assessed at Week 10 (2 weeks after the last dose of study drug) and the secondary efficacy endpoint will be assessed at Week 6.
[0351] rhuMAbβ7 100 mg SC (fixed dose) at Weeks 0, 4 and 8 and 420 mg SC at Week 0 (flat loading dose) followed by 300 mg SC or matching placebo SC at Weeks 2, 4 and 8 within a dose range of 1:1: Patients were randomized in a ratio of 1. Researc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com