Combination of EPA, DPA and/or DHA with a chemotherapeutic agent
A chemotherapeutic agent, inhibitor technology, applied in the field of combined use of EPA, DPA and/or DHA and chemotherapeutic agents, can solve the problem of no disclosure of EPA and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0097] Total Fatty Acid Analysis
[0098] Murine C26 adenocarcinoma cells were routinely cultured twice a week, and cell cultures were studied with binoculars to determine cell confluency. The medium was removed with a vacuum aspirator and the cells were washed once with 10 ml of pre-warmed PBS. PBS was removed and 2 ml of trypsin-EDTA was added to the culture flask. After a short incubation in the incubator, cells were studied with binoculars to determine cell detachment. The cells were thoroughly resuspended in 8 ml medium. Cells were passaged in new flasks at a ratio of 1:3 or 1:5, depending on the confluence of the cell culture. Additionally, 25 ml of fresh medium was added to the bottle.
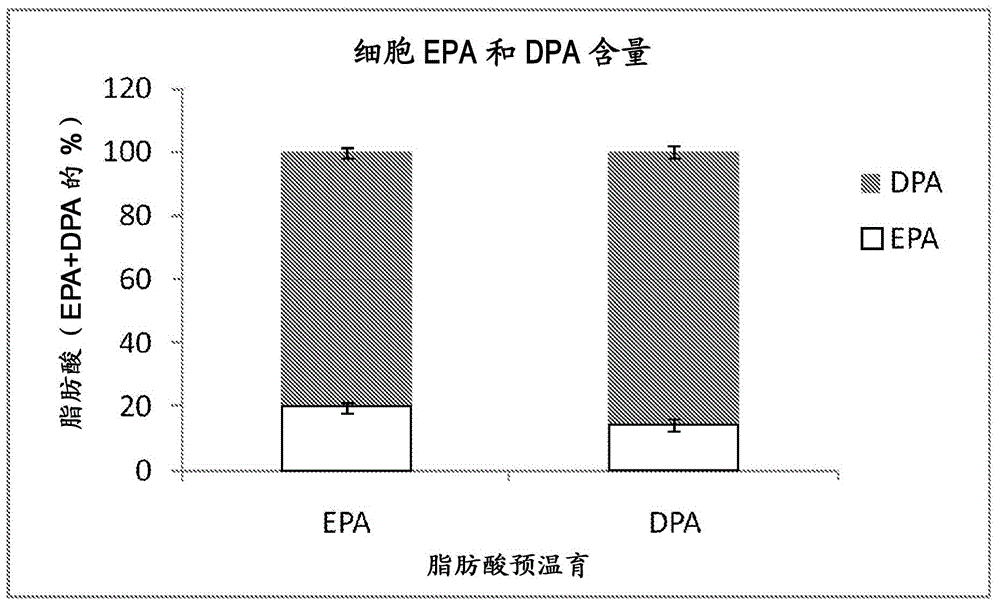
[0099] To determine total phospholipid fatty acids, murine C26 cells were cultured in 6-well plates. After 4 days of incubation with 50 μM of EPA, DPA or DHA, cells were washed with pre-warmed PBS and trypsinized. After detachment, cells were resuspended in 1 ml medium and transfe...
Embodiment 2
[0102] Metabolic activity of EPA, DHA or DPA
[0103] C26 cells were pre-incubated with EPA, DHA or DPA for 4 days prior to chemotherapy incubation at a final concentration of 50 μM. To avoid direct interaction between fatty acids and chemotherapeutic drugs, media containing fatty acids was removed prior to the addition of chemotherapeutic drugs. Cells were incubated with the chemotherapeutic drugs cisplatin and doxorubicin for 24 hours, and their metabolic activity was measured after incubation. Figure 4 shows the results of metabolic activity of chemotherapy-treated cells pre-incubated with EPA, DHA or DPA. Addition of EPA or DPA resulted in a significant decrease in metabolic activity after cisplatin treatment (p<0.0001) as well as after doxorubicin treatment (p<0.0001). DHA did not show significant changes in metabolic activity compared to controls.
Embodiment 3
[0105] skeletal muscle function
[0106] 5–6 week-old male CD2F1 mice (BALB / c x DBA / 2, Harlan / Charles River, The Netherlands) were housed individually in a climate-controlled room (constant 21±1°C room temperature, 12:12 light-dark cycle). After one week of acclimation, the mice were divided into weight-matched groups: (1) the control group received the control diet (C), (2) the tumor-bearing group received the control diet (TB) and (3) the tumor-bearing group received the same as before The Specific Nutrient Combination (TB-SNC) diet containing high protein, leucine and fish oil content as active ingredients with the addition of a specific oligosaccharide mixture [1]. All experimental procedures were approved by the animal ethics committee (DEC consultants, Bilthoven, The Netherlands) and followed the principles of good laboratory animal care.
[0107] The muscle contractile properties of the extensor digitorum longus (EDL) muscle of 7–9-week-old male CD2F1 mice were evaluat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com