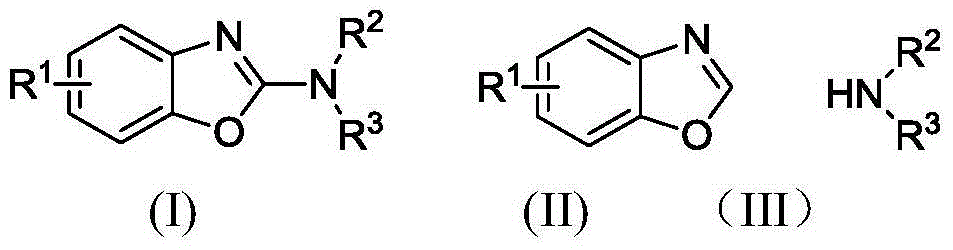Electrochemical catalytic synthesis method of 2-N-substituted benzoxazole compounds
A technology of benzoxazole and 2-N-, which is applied in the field of electrochemical catalytic synthesis of 2-N-substituted benzoxazole compounds, can solve the problems of complex operation, waste of co-oxidant, and low solubility, and achieve The post-treatment operation is simple, the reaction conditions are mild, and the effect of avoiding a large amount of use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1: Electrochemically catalyzed 2-N-morpholino amination of benzoxazole
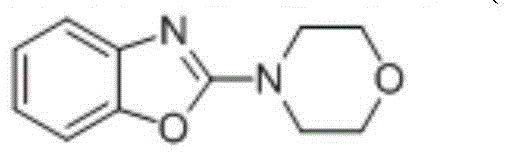
[0024] In a 50mL single-chamber electrolytic cell, dissolve benzoxazole (1.0mmol) in acetonitrile, then add glacial acetic acid (5mmol), and add morpholine (2.0mmol) and tetrabutylammonium iodide ( 0.1mmol), with the glassy carbon electrode as the anode and the iron sheet as the cathode, at 6mA / cm 2 electrolysis at a constant current. When the reaction of the raw materials was completed, the electrolysis was stopped, the solvent was removed, dissolved in dichloromethane, washed three times with saturated sodium carbonate solution, and separated by column chromatography to obtain 2-(4-morpholino)benzoxazole. Yield: 91%.
[0025]
[0026] White solid;86℃; 1 H NMR (400MHz, CDCl 3 ):3.70-3.72(m,4H)3.83-3.85(m,4H),7.06(td,J=7.8,1.1Hz,1H),7.06(td,J=7.6Hz,1.0Hz,1H),7.23- 7.30(m,1H),7.38-7.40(m,1H).
Embodiment 2
[0027] Example 2: Electrochemically catalyzed 2-N-morpholino amination of benzoxazole
[0028] In a 50mL single-chamber electrolytic cell, dissolve benzoxazole (1.0mmol) in acetonitrile, then add glacial acetic acid (5mmol), and add morpholine (2.0mmol) and tetrabutylammonium iodide ( 0.2mmol), with the glassy carbon electrode as the anode and the iron sheet as the cathode, at 6mA / cm 2 electrolysis at a constant current. When the reaction of the raw materials was completed, the electrolysis was stopped, the solvent was removed, dissolved in dichloromethane, washed three times with saturated sodium carbonate solution, and separated by column chromatography to obtain 2-(4-morpholino)benzoxazole. Yield: 87%.
Embodiment 3
[0029] Example 3: Electrochemically catalyzed 2-N-morpholino amination of benzoxazole
[0030] In a 50mL single-chamber electrolytic cell, dissolve benzoxazole (1.0mmol) in acetonitrile, then add glacial acetic acid (5mmol), and add morpholine (2.0mmol) and tetrabutylammonium iodide ( 0.1mmol), with graphite electrode as anode and iron sheet as cathode, at 6mA / cm 2 electrolysis at a constant current. When the reaction of the raw materials was completed, the electrolysis was stopped, the solvent was removed, dissolved in dichloromethane, washed three times with saturated sodium carbonate solution, and separated by column chromatography to obtain 2-(4-morpholino)benzoxazole. Yield: 77%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com