Omeprazole and sodium bicarbonate core-covering tablet
A technology of sodium bicarbonate and omeprazole, which is applied in the field of packaged chips containing active ingredients of omeprazole and sodium bicarbonate, can solve problems such as affecting qualitative and quantitative analysis results, disorderly disintegration, and reducing effects, and achieves Easy inspection and quality control, reduced degradation products, improved stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
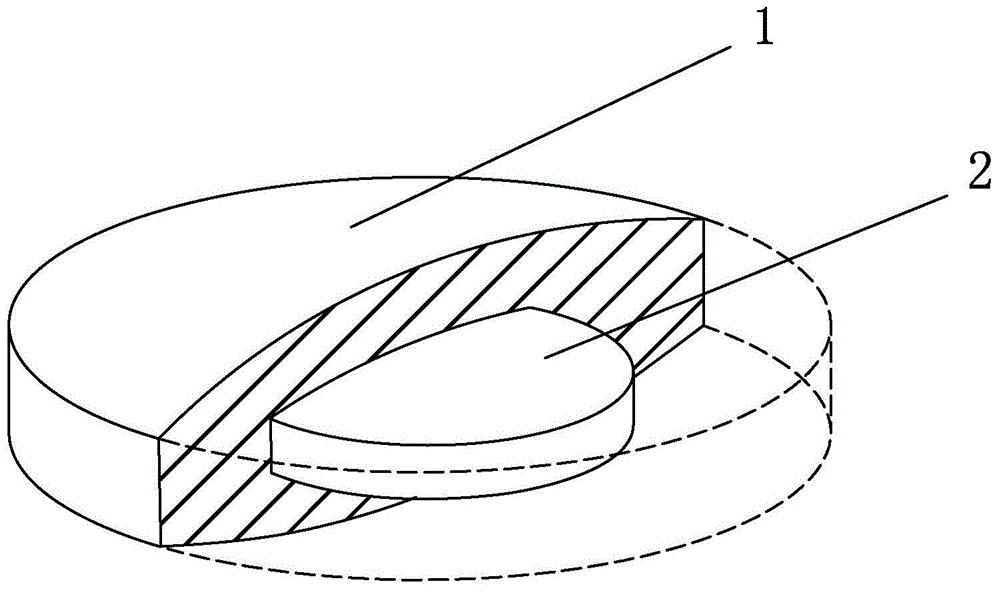
[0028] Embodiment 1: as figure 1 As shown in the omeprazole sodium bicarbonate core-coated tablet A, the drug-containing tablet core 2 contains 6.7 mg omeprazole, and the peripheral drug-containing layer 1 contains 366.7 mg of sodium bicarbonate and contains immediate-release auxiliary materials. The tablet core 2 and The drug-containing layers 1 are all circular.
Embodiment 2
[0029] Embodiment 2: Omeprazole sodium bicarbonate core-coated B sheet is substantially the same as Example 1, and the difference is that the drug-containing tablet core contains 13.3mg omeprazole, and the drug-containing layer on the periphery contains 366.7mg of sodium bicarbonate and Contains sustained-release excipients.
Embodiment 3
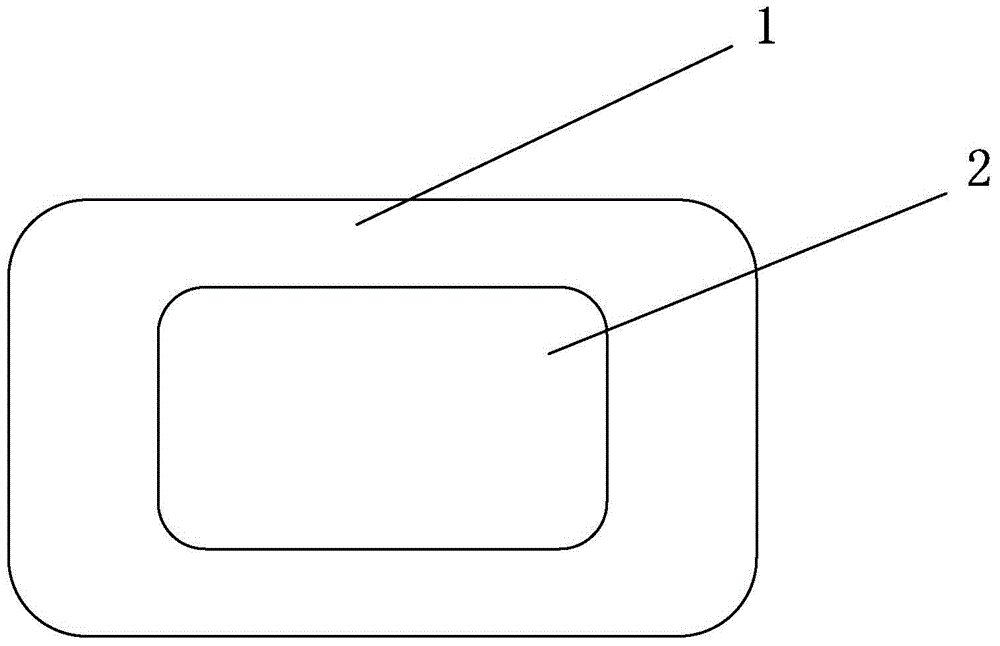
[0030] Embodiment 3: as figure 2 The shown omeprazole sodium bicarbonate core-coated tablet C is a rectangle with rounded corners. The drug-containing tablet core 2 contains 10 mg of omeprazole, and the drug-containing layer 1 on the periphery contains 550 mg of sodium bicarbonate and contains immediate-release excipients.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com