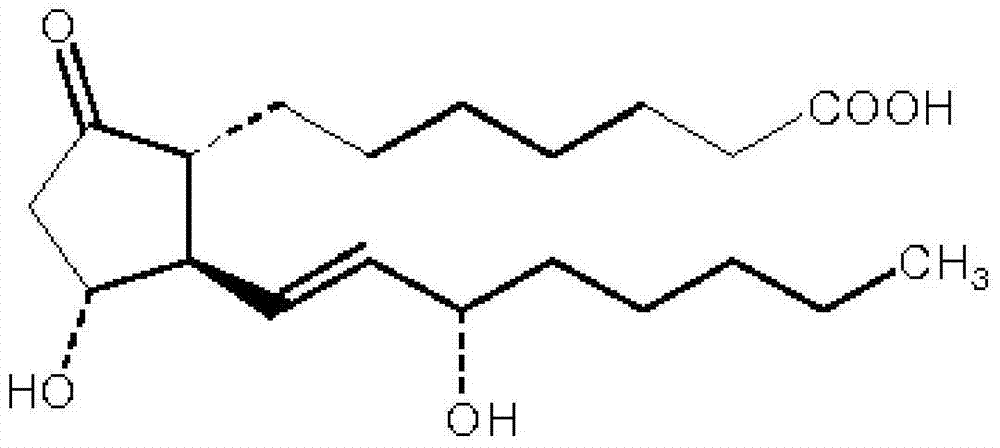
Alprostadil injection and preparation method thereof
The technology of dier injection and alprostadil is applied in the direction of medical formula, medical preparation containing active ingredients, drug delivery, etc. It can solve the problems of demulsification, inconvenient operation, difficult filtration, etc., and achieve less related substances, The effect of good stability and high content of the main drug
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
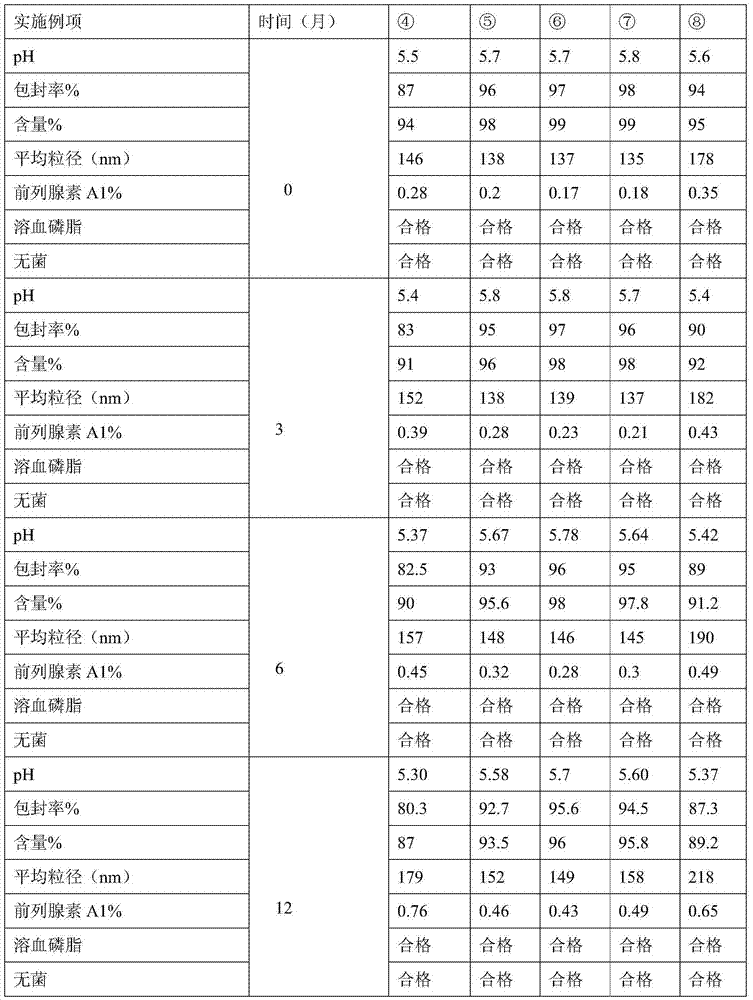
Examples
Embodiment 1
[0028] 1) Add 18g of lecithin, 2.4g of oleic acid and 5mg of alprostadil into 100g of soybean oil, heat to 70±2°C and stir to dissolve, as the oil phase;
[0029] 2) Add 22.5 g of isotonic agent glycerin and pH regulator sodium hydroxide to water, heat to 65±2°C, stir and mix, filter, and use as water phase, the volume of the prepared water phase is 9 times that of the oil phase;
[0030] 3) Stir and mix the water phase and the oil phase to form colostrum, and add water for injection to 1000ml. Then homogenize under high pressure, filter and sterilize.
Embodiment 2
[0032] 1) Add 18g of lecithin, 2.4g of oleic acid and 5mg of alprostadil into 100g of soybean oil, heat to 70±2°C and stir to dissolve, as the oil phase;
[0033] 2) Add 22.5g of isotonic agent glycerin and pH regulator sodium hydroxide into water, heat to 65±2°C, stir and mix, filter, and use as water phase, the volume of the prepared water phase is 8 times that of the oil phase;
[0034] 3) Stir and mix the water phase and the oil phase to form colostrum, and add water for injection to 1000ml. Then homogenize under high pressure, filter and sterilize.
Embodiment 3
[0036] 1) Add 18g of lecithin, 2.4g of oleic acid and 5mg of alprostadil into 100g of soybean oil, heat to 70±2°C and stir to dissolve, as the oil phase;
[0037] 2) Add 22.5g of isotonic agent glycerin and pH regulator sodium hydroxide to water, heat to 65±2°C, stir and mix, filter, and use it as the water phase, the volume of the prepared water phase is 7 times that of the oil phase;
[0038] 3) Stir and mix the water phase and the oil phase to form colostrum, and add water for injection to 1000ml. Then homogenize under high pressure, filter and sterilize.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com