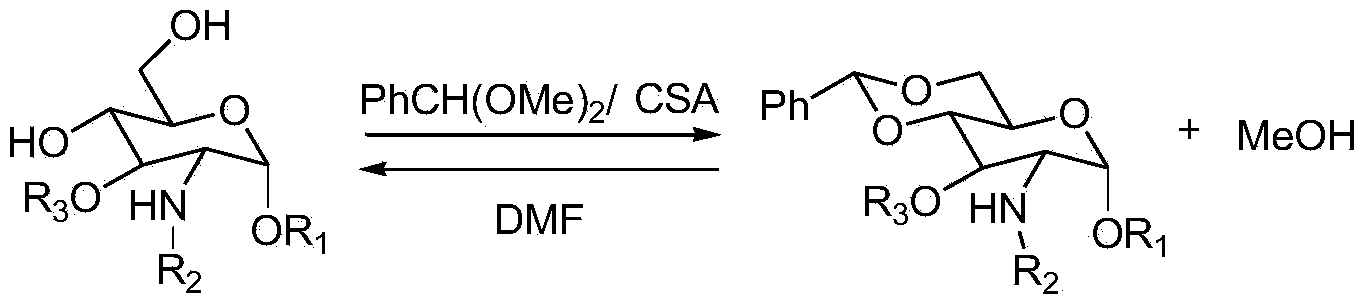
Method for protecting D-glucosamine derivative by using benzaldehyde dimethyl acetal
A technology of benzaldehyde dimethyl acetal and glucosamine, which is applied to the preparation of sugar derivatives, sugar derivatives, sugar derivatives, etc., can solve the problems of complex post-processing, poor raw material utilization, and low reaction conversion rate, and achieve Simplify the post-treatment process, increase the reaction conversion rate, and reduce the cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] The 250mL four-neck bottle is equipped with mechanical stirring, thermometer and condenser. Add D-glucosamine derivative (10.0g, 29.3mmol, 1.0eq), camphorsulfonic acid (0.18g, 0.77mmol, 0.026eq), benzaldehyde dimethyl acetal (9.7g, 61.8mmol, 2.0eq), tetrahydrofuran (100mL), heated to reflux. Stir for 2 hours with heat preservation, during which 80 mL of condensed tetrahydrofuran was separated out, and 85 mL of tetrahydrofuran was added to keep the volume in the reaction bottle unchanged. After the reaction was completed, the temperature was lowered to room temperature, 50 mL of purified water (0.09 g of sodium bicarbonate was dissolved in it) was added to the reaction flask, and the reaction flask was incubated and stirred in an ice-water bath at 3°C for 2 hours. After filtering, the filter cake was washed twice with 20 mL of methanol*2 to obtain 11.3 g of white solid, which was dried in vacuum to obtain 10.8 g of white solid powder. The normalized content is 99.60%...
Embodiment 2
[0021] The 500mL four-necked bottle is equipped with a mechanical stirrer, a thermometer and a condenser. Add D-glucosamine derivative (10.0g, 29.3mmol, 1.0eq), camphorsulfonic acid (0.68g, 2.93mmol, 0.1eq), benzaldehyde dimethyl acetal (10.3g, 64.5mmol, 2.2eq), acetonitrile (300mL), heated to reflux. Insulated and stirred for 6 hours, during which a total of 350 mL of condensed acetonitrile was separated out, and 380 mL of acetonitrile was added to keep the volume in the reaction bottle constant. After the reaction is completed, desolvate under reduced pressure until 150 mL of the reaction liquid remains in the reaction bottle, add 50 mL of purified water into the reaction bottle, and keep stirring in an ice-water bath at 3°C for 2 hours. After filtering, the filter cake was washed twice with 20 mL of methanol*2 to obtain 11.0 g of white solid, which was dried in vacuum to obtain 10.3 g of white solid powder. The normalized content is 98.99%, and the normalized yield is 8...
Embodiment 3
[0025] Put substrate (1.50kg, 4.44mol, 1.0eq), camphorsulfonic acid (26.1g, 0.11mol, 0.025eq) and benzaldehyde dimethyl acetal (1.37kg, 8.88mol, 2.0eq) into the 30L jacketed kettle in sequence , THF (13.4 kg). Reflux for 8 hours, during which a total of 13.1 kg of condensed tetrahydrofuran was separated out, and 13.5 kg of tetrahydrofuran was added to keep the reaction volume constant. After the reaction is over, distill under reduced pressure until the remaining reaction liquid in the kettle is about 8L, transfer tap water (8.0kg) into the reaction kettle, and lower the temperature to 0°C-5°C. After insulated and stirred for 2 hours, centrifuge. The filter cake was washed with cold methanol (4kg, 0°C-5°C). Finally, 1.98 kg of slightly yellowish white filter cake was obtained. After drying at 45°C, 1.68kg of white solid powder was obtained, the normalized content was 98.22%, and the normalized yield was 89.43%.
[0026] A total of 13.1 kg of THF was extracted, 500 g of cal...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com