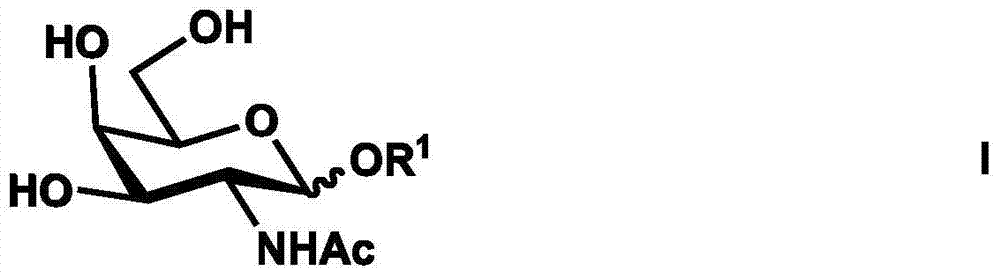Synthetic method of double-sialylated tetrasaccharide
A synthesis method and sialylation technology, applied in chemical instruments and methods, organic chemistry, sugar derivatives, etc., can solve problems such as indistinguishable
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
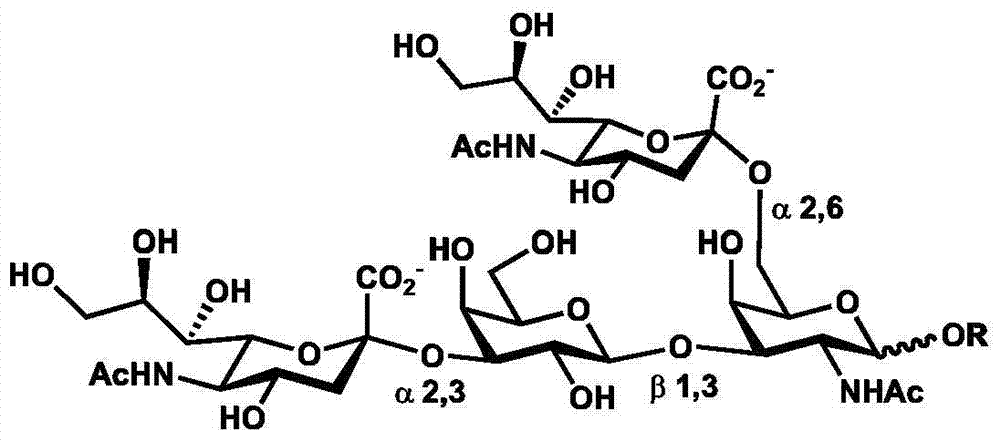
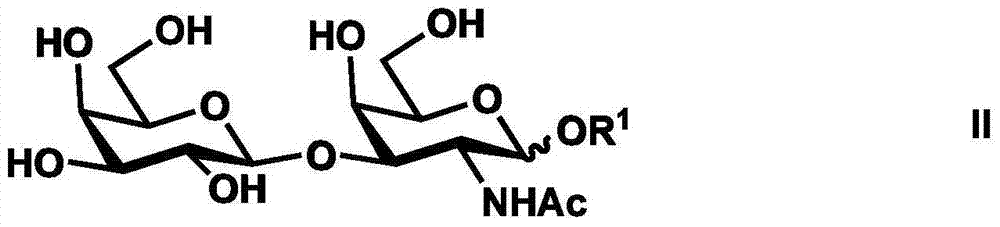
[0049] Example 1 Chemical enzymatic synthesis of disialylated tetrasaccharide [Neu5Acα(2-3)Galβ(1-3)[Neu5Acα(2-6)]GalNAc]
[0050] Proceed as follows:
[0051] (1) Synthesis of β-configuration monosaccharide derivative 1 by chemical method:
[0052] Compound 1 (GalNAcβProN 3 )Synthesis
[0053] The reaction equation is as Figure 8 shown;
[0054] In a 500mL round bottom flask, add galactosamine hydrochloride (8.0g, 37.1mmol), acetic anhydride (38mL), pyridine (160mL), dimethylaminopyridine (DMAP, 0.3g, 2.46mmol), and stir at room temperature for 12 Hour. Thin-layer chromatography detection (EA:MeOH=10:1) After the reaction is complete, concentrate by rotary evaporation, then add 20 mL of toluene to the reaction solution, concentrate by rotary evaporation, and repeat 3 times. The obtained solid was redissolved in 300 mL of methanol, and left to stand at 4° C. for 12 hours for recrystallization. After filtration, the filtrate was discarded, and the resulting solid was co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com