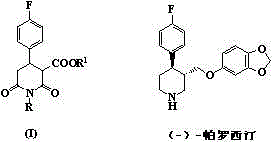
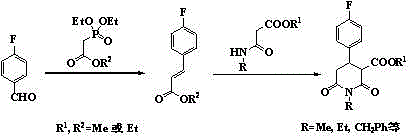
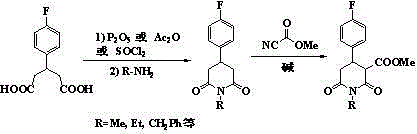
Synthetic process of 1-alkyl-4-p-fluorophenyl-2,6-piperadinedione-3-formic ester
A technology of p-fluorophenyl and diketone, which is applied in the field of chemical synthesis, can solve problems such as complex process routes, high cost, and large pollution, and achieve the effects of high product yield, low production cost, and less three wastes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030]33.7g (0.1mol) of 1-methyl-4-p-fluorophenyl-2,6-piperidinedione-3,5-dicarboxylate 33.7g (0.1mol) and methanol 280g were stirred and mixed evenly, and the concentration of 19% hydrogen was added dropwise 150g of sodium aqueous solution, heated to 70°C for 5 hours after dripping, cooled to below 10°C, added 400g of water, continued to stir and cooled to below 10°C, filtered and washed to obtain 6.2g of unreacted raw materials, 5.5g in dry weight, which was 16.3 %. The filtrate was acidified to pH 2.0 by adding 2.0mol / L hydrochloric acid, and an off-white solid was precipitated, filtered, washed with water, and dried in vacuum to obtain the intermediate 1-methyl-4-p-fluorophenyl-2,6-piperidinedione-3- 25.4 g of formic acid-5-methyl carboxylate. The intermediate was dissolved in 200 g of methyl isobutyl ketone, 0.2 g of copper oxide was added, and the temperature was slowly raised to 110° C. for decarboxylation. After 7 hours, the decarboxylation was completed. Stir and co...
Embodiment 2
[0032] Stir and mix 36.5g (0.1mol) of diethyl 1-methyl-4-p-fluorophenyl-2,6-piperidinedione-3,5-dicarboxylate and 300g of isopropanol evenly, dropwise at a concentration of 22 % sodium hydroxide aqueous solution 140g, after dripping, heat up to 65°C for 8 hours, cool to below 10°C, add 400g of water, continue to stir and cool to below 10°C, filter and wash to obtain 5.8g of unreacted raw materials, 5.2g in dry weight, as 14.2% of the feed. The filtrate was acidified to pH 2.0 by adding 2.0mol / L hydrochloric acid, and a light yellow solid was precipitated, filtered, washed with water, and dried in vacuum to obtain the intermediate 1-methyl-4-p-fluorophenyl-2,6-piperidinedione-3- Formic acid-5-ethyl carboxylate 27.0g. The intermediate was dissolved in 200 g of butyl acetate, 0.4 g of zinc oxide was added, and the temperature was slowly raised to 120° C. for decarboxylation. After 6 hours, the decarboxylation was completed. Stir and cool to below 30°C, filter and wash, add wate...
Embodiment 3
[0034] Mix 41.3g (0.1mol) of dimethyl 1-benzyl-4-p-fluorophenyl-2,6-piperidinedione-3,5-dicarboxylate and 260g of methanol evenly, and add hydrogen at a concentration of 21% dropwise Sodium oxide aqueous solution 155g, after dripping, heat up to 75°C for 6 hours, cool to below 10°C, add 400g of water, continue to stir and cool to below 10°C, filter and wash with water to obtain 8.2g of unreacted raw materials, 7.1g in dry weight, which is the raw material for feeding 17.2%. The filtrate was acidified to pH 2.0 by adding 2.0mol / L hydrochloric acid, and a slightly brown solid was precipitated, filtered, washed with water, and dried in vacuum to obtain the intermediate 1-benzyl-4-p-fluorophenyl-2,6-piperidinedione-3- 31.2 g of methyl formic acid-5-carboxylate. The intermediate was dissolved in 200 g of diethyl carbonate, 0.4 g of cuprous oxide was added, and the temperature was slowly raised to 100° C. for decarboxylation. After 10 hours, the decarboxylation was completed. Stir...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com