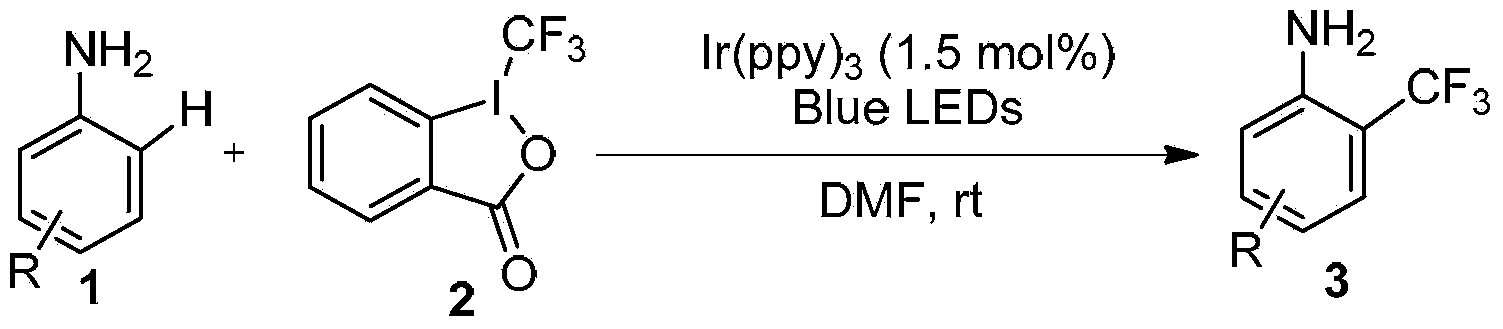
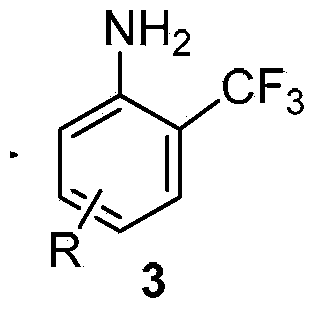
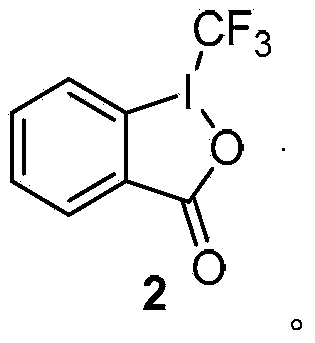
Method for preparing o-trifluoromethyl phenylamine and derivatives thereof
A technology of trifluoromethylaniline and aniline derivatives, which is applied in the field of preparation of trifluoromethylaniline derivatives, can solve the problem of less trifluoromethylaniline, and achieve the effect of mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Under the protection of nitrogen or argon, 4-bromoaniline 0.4mmol, 0.2mmol, Ir(ppy) 3 (2 mg) and 1 ml of DMF were added to the reaction flask, and then irradiated with a blue LED light strip (7W) at room temperature until the trivalent iodine reagent was completely converted to complete the reaction. Add 10 mL of saturated Na 2 CO 3 aqueous solution, extracted 3 times with ethyl acetate, the organic layer was washed once with saturated brine, anhydrous Na 2 SO 4 Dry the organic layer. Column chromatography [200-300 mesh chromatography silica gel (the same below), eluent: petroleum ether 60-90: ethyl acetate = 20:1-10:1], to obtain the product Yield 61% (86% NMR yield). 1 H NMR (400MHz, CDCl 3 ):δ(ppm)=7.53(d,J=2.0Hz,1H),7.37(dd,J=8.8,2.0Hz,1H),6.63(d,J=8.0Hz,1H),3.86(s,2H ); 13 C NMR (100MHz, CDCl 3 ):δ(ppm)=143.5,135.6,129.2(q,J=5.5Hz),124.0(q,J=270.7Hz),110.8,115.2(q,J=30.9Hz); 19 F NMR (376MHz, CDCl 3 ):δ(ppm)=-63.2; HRMS(ESI)m / z calcd for C 7 h 4 B...
Embodiment 2
[0022] Under the protection of nitrogen or argon, 4-iodoaniline 0.4mmol, 0.2mmol, Ir(ppy) 3 (2 mg) and 1 ml of DMF were added to the reaction flask, and then irradiated with a blue LED light strip (7W) at room temperature until the trivalent iodine reagent was completely converted to complete the reaction. Add 10 mL of saturated Na 2 CO 3 aqueous solution, extracted 3 times with ethyl acetate, the organic layer was washed once with saturated brine, anhydrous Na 2 SO 4 Dry the organic layer. Column chromatography (eluent: petroleum ether 60-90: ethyl acetate = 20:1-10:1) to obtain the product Yield 60%. 1 H NMR (400MHz, CDCl 3 ):δ(ppm)=7.68(d,J=1.6Hz,1H),7.52(dd,J=8.4,1.6Hz,1H),6.51(d,J=8.4Hz,1H),3.98(s,2H ); 13 C NMR (100MHz, CDCl 3 ):δ(ppm)=144.1,141.4,134.9(q,J=5.4Hz),123.8(q,J=271.3Hz),119.2,115.7(q,J=30.8Hz),77.4; 19 F NMR (376MHz, CDCl 3 ):δ(ppm)=-63.2; HRMS(ESI)m / z calcd for C 7 h 4 f 3 IN[M-H] - :285.9346;found:285.9341.
Embodiment 3
[0024] Under the protection of nitrogen or argon, 0.4mmol, 0.2mmol, Ir(ppy) 3 (2 mg) and 1 ml of DMF were added to the reaction flask, and then irradiated with a blue LED light strip (7W) at room temperature until the trivalent iodine reagent was completely converted to complete the reaction. Add 10 mL of saturated Na 2 CO 3 aqueous solution, extracted three times with ethyl acetate, washed once with saturated brine, anhydrous Na 2 SO 4 Dry the organic layer. Column chromatography (eluent: petroleum ether 60-90: ethyl acetate = 15:1-8:1) to obtain the product Yield 67%. 1 H NMR (400MHz, CDCl 3 ):δ(ppm)=8.14(d,J=1.6Hz,1H),7.95(dd,J=8.4,1.6Hz,1H),6.73(d,J=8.4Hz),4.31(s,2H), 3.88(s,3H); 13 C NMR (100MHz, CDCl 3 ):δ(ppm)=166.2,148.3(d,J=1.8Hz),134.3,129.2(q,J=4.8Hz),124.5(q,J=271.2Hz),119.1,116.3,112.7(q,J =30.8Hz),51.9; 19 F NMR (376MHz, CDCl 3 ):δ(ppm)=-63.1; HRMS(ESI)m / z calcd for C 9 h 7 f 3 NO 2 [M-H] - :218.0434;found:218.0449.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com