Improved dimethicone-containing injection
A technology of simethicone and injection, which is applied in medical preparations with non-active ingredients, medical preparations containing active ingredients, liquid delivery, etc. It can solve problems such as simethicone residue and achieve good redispersibility, Small irritation, good suspension of the preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1, preparation 10% doxycycline hydrochloride injection
[0035] Preparation composition: Each 100ml injection contains doxycycline hydrochloride 10g, SAIB 17g, viscosity 5cs simethicone 25ml, IPM added to 100ml.
[0036] Preparation method: (1) Dissolving SAIB in IPM to prepare SAIB / IPM solution; (2) Mixing doxycycline hydrochloride and simethicone oil, grinding through a colloid mill until the particle size of doxycycline is less than 10 μm, and then mixing with SAIB / IPM is mixed and homogenized several times with a high-shear homogenizer at 15,000-21,000 rpm to prepare an injection with a particle size of less than 10 μm.
Embodiment 2
[0037] Embodiment 2, preparation 18% doxycycline hydrochloride injection
[0038] Preparation composition: Each 100ml injection contains doxycycline hydrochloride 18g, SAIB 20g, aluminum stearate 0.4g, BHA 0.01g, BHT 0.01g, viscosity 5cs simethicone 10ml, IPM added to 100ml.
[0039] Preparation method: (1) Add aluminum stearate to IPM, stir and heat (95-100°C) to prepare aluminum stearate-containing oil gel; (2) Dissolve SAIB in oil gel to prepare SAIB / Oil gel solution; (3) Doxycycline hydrochloride is mixed with oil gel, and the particle size of doxycycline hydrochloride is less than 8 μ m through sand mill grinding, then add simethicone and antioxidant, and use high shear After homogenization by the homogenizer several times under the condition of 15000-21000rpm, an injection with a particle size of less than 8 μm is prepared.
Embodiment 3
[0040] Embodiment 3, stability test and blood drug concentration determination
[0041] (1) Sedimentation rate and redispersibility: the sedimentation volume ratio of the preparation of Example 2 after standing for 3 hours was 94%; the redispersibility test results showed that the preparation was easily dispersed after shaking, and there was no sediment at the bottom of the bottle.
[0042] (2) Stability test: the formulation of Example 2 was sealed in a 20ml ampoule and stored in the dark at 28-32°C for 6 months. The sedimentation volume ratio of the formulation was 68-76%, and it was easily dispersed after shaking. Precipitate. The doxycycline content in the preparation was reduced by 1.63-2.75% (percentage of initial amount).
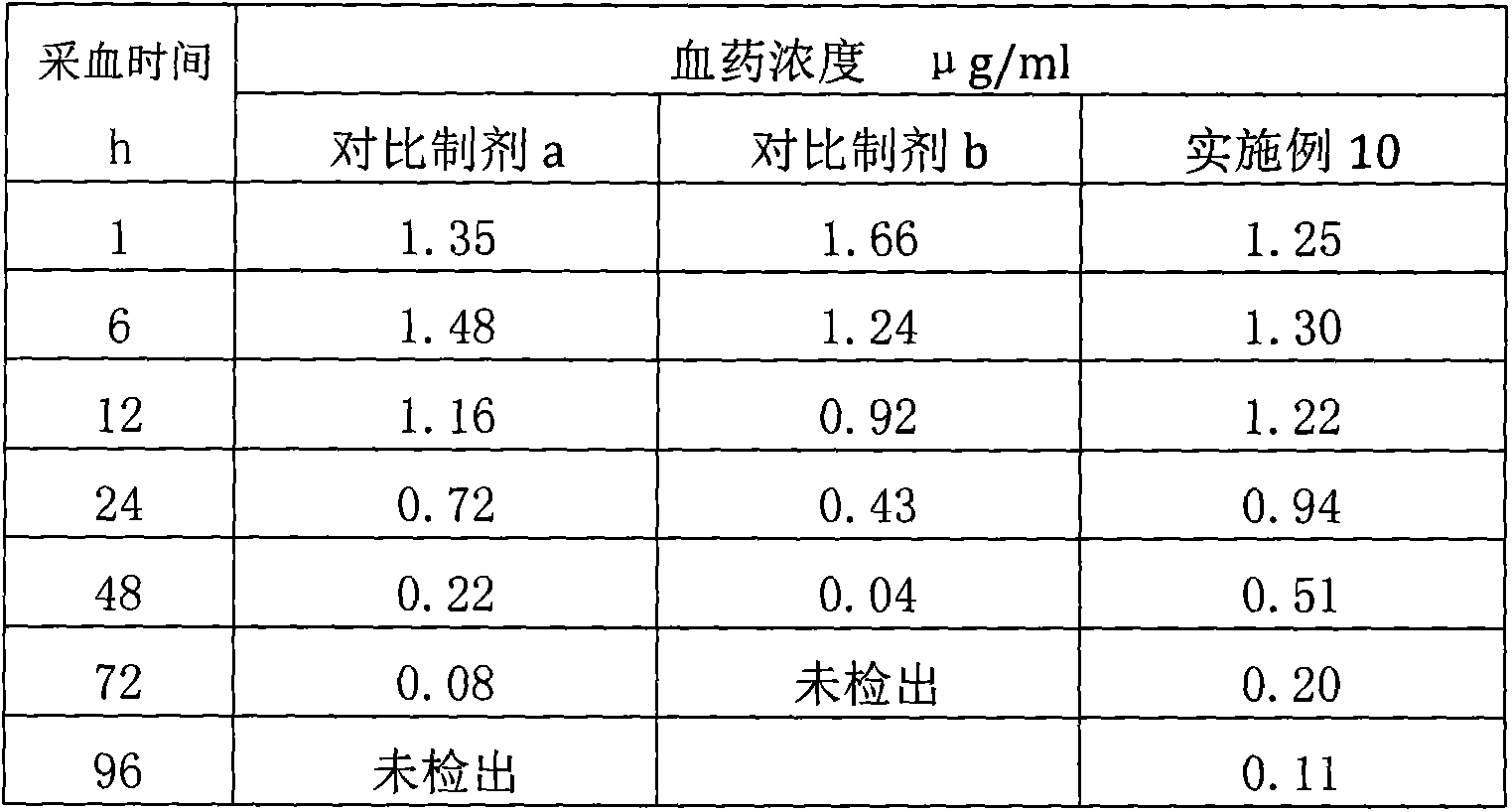
[0043] (3) Determination of blood drug concentration: choose 15 healthy pigs with a body weight of 25-30 kg, divide them into three groups, 5 pigs in each group, inject the preparation of Example 2 intramuscularly respectively, and the dosage is 10 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com