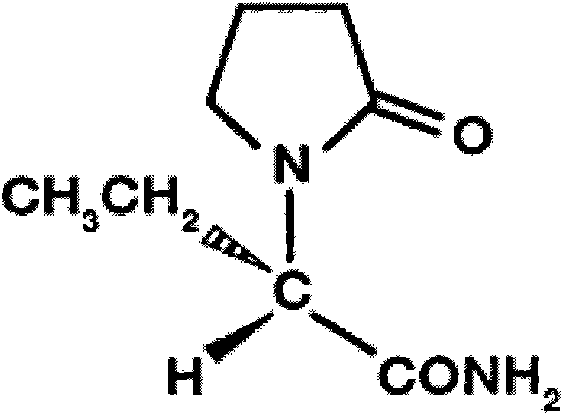
Preparation method of levetiracetam injection
A technology for injection and preparation process, which is applied in the field of medicine, can solve the problems of inability to guarantee the clinical safety of levetiracetam injection, failure to achieve sterility, etc., and achieve the effect of stable product quality and simple production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
5000.0
[0043] The preparation method is to weigh about 4000g of water for injection below 30°C, add the prescribed amount of sodium acetate trihydrate and sodium chloride, stir and dissolve completely; add the prescribed amount of levetiracetam raw material drug into the above solution a, stir Dissolve; adjust the pH value of the solution to 5.0-6.0 with glacial acetic acid; add water for injection to 5000mL; filter through a 0.22μm improved PVDF membrane, fill after filtration, and sterilize at 121°C for 15min to obtain levetirazil per 1mL Levetiracetam injection 100mg.
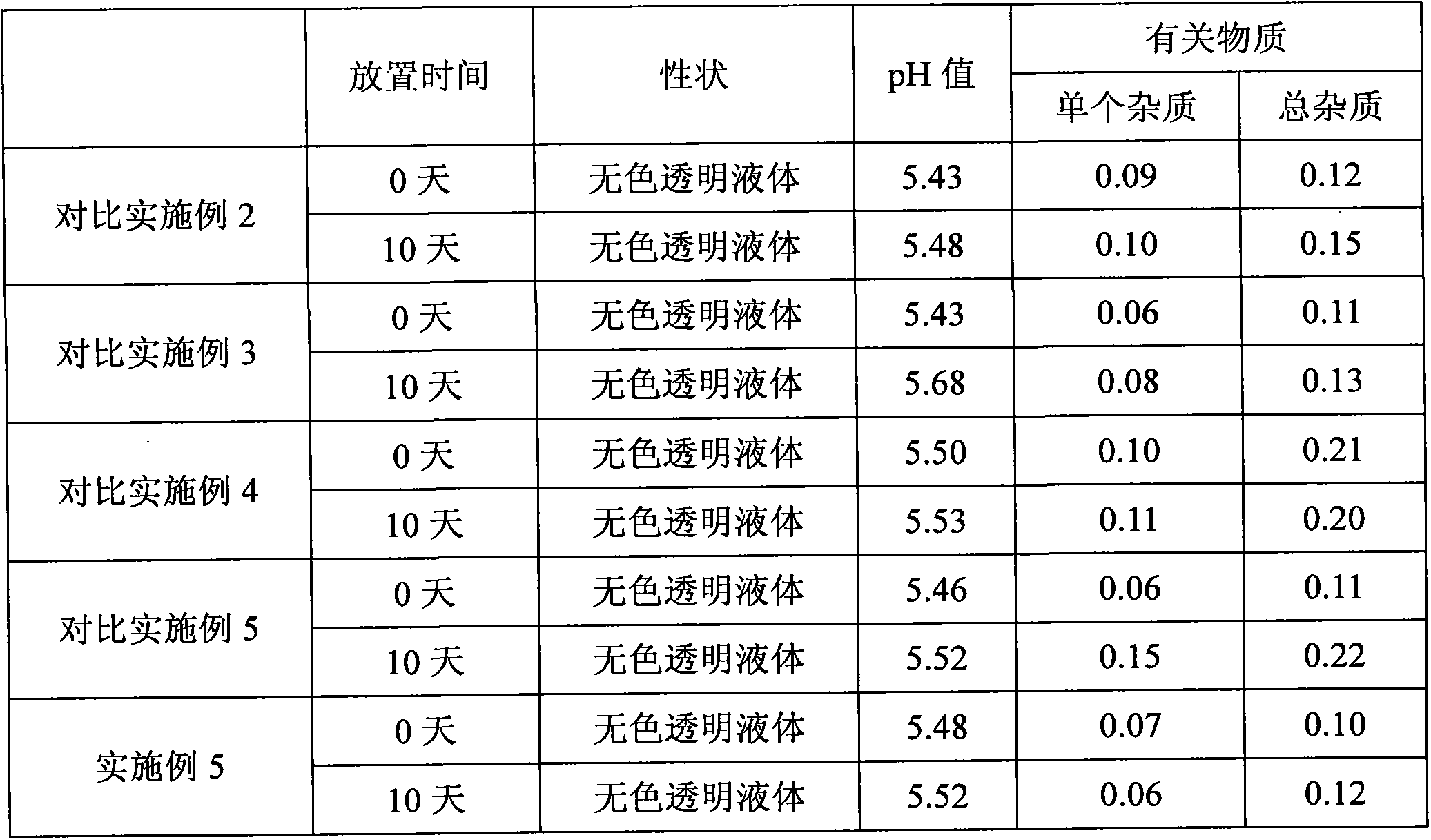
[0044] According to Chinese Pharmacopoeia Appendix XI E bacterial endotoxin detection method, detect the bacterial endotoxin of Comparative Example 1 and Example 1 levetiracetam injection, the results are compared with Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com