Polylactic acid-polyethylene glycol-tumor penetrating peptide compound, preparation and application thereof
A technology of tumor-penetrating peptides and polyethylene glycol, which is applied in the field of targeted polymer materials with tumor-penetrating properties and its preparation and application, and can solve the problems of insignificant improvement of drug efficacy, iNGR polypeptide-targeting materials, and tumor Poor targeting performance and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
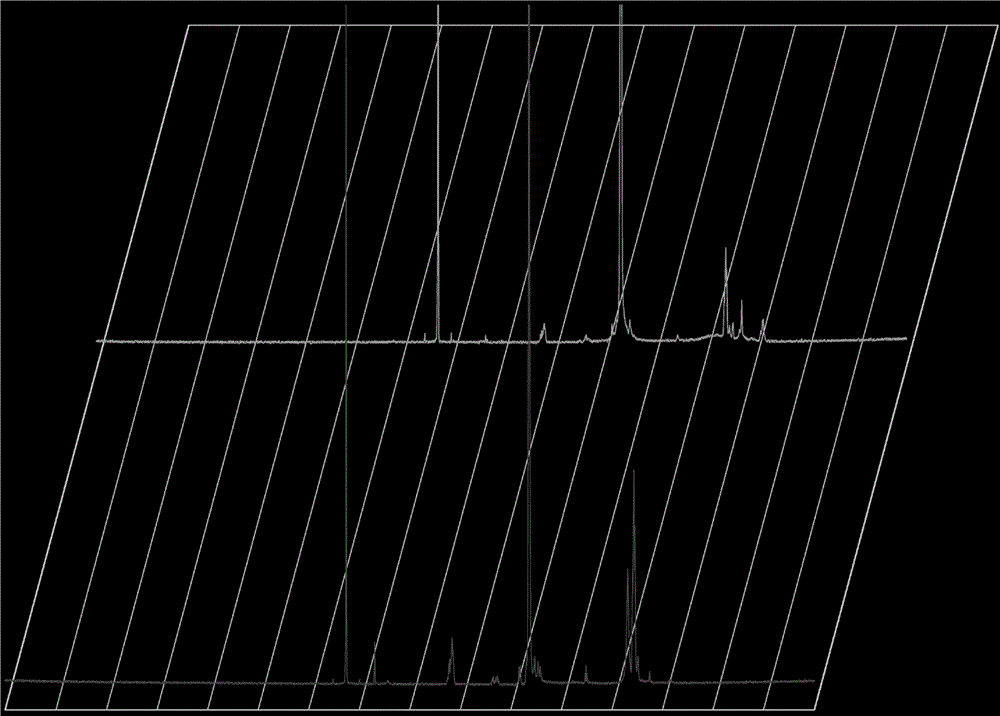
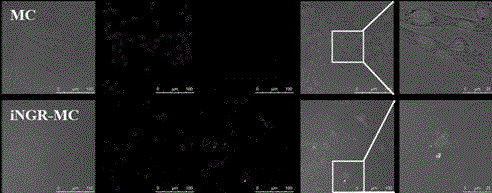
[0023] Example 1: Synthesis, purification and characterization of the complex PLA-PEG-iNGR.
[0024] Deprotect the 4-methyldiphenylmethylamine resin (MBHA) with trifluoroacetic acid (TFA) for 1 minute, twice, dissolve the Boc-protected amino acid in 0.5M HBTU (the solvent is DMF), and react at room temperature for 20 minutes. Wash with DMF, remove Boc protection with TFA, and connect all amino acids sequentially according to the amino acid sequence. After the reaction, wash the resin, remove the protective group with TFA, and dry it in vacuum. Then put it into a peptide cutting tube, add an appropriate amount of P-cresol, then pass it into HF, stir and react in an ice bath for 1 hour; after the reaction, remove the HF in the tube under reduced pressure. , the residue was precipitated with an appropriate amount of glacial ether, filtered to obtain the precipitate and washed with glacial ether; the precipitate was re-dissolved with TFA, and filtered to obtain the filtrate; ) (s...
Embodiment 2
[0025] Example 2: Synthesis, purification and characterization of the complex PLA-PEG-iNGR.
[0026] Deprotect 4-methyldiphenylmethylamine resin (MBHA) with trifluoroacetic acid (TFA) for 1 minute, twice, dissolve Boc-protected amino acid in 0.5M HBTU (solvent is DMF), react at room temperature for 25 minutes, Wash with DMF, remove Boc protection with TFA, and connect all amino acids sequentially according to the amino acid sequence. After the reaction, wash the resin, remove the protective group with TFA, and dry it in vacuum. Then put it into a peptide cutting tube, add an appropriate amount of P-cresol, then pass it into HF, stir and react in an ice bath for 1 hour; after the reaction, remove the HF in the tube under reduced pressure. , the residue was precipitated with an appropriate amount of glacial ether, filtered to obtain the precipitate and washed with glacial ether; the precipitate was re-dissolved with TFA, and filtered to obtain the filtrate; ) (sequence C-C(Acm)...
Embodiment 3
[0027] Example 3: Synthesis, purification and characterization of the complex PLA-PEG-iNGR.
[0028]Deprotect the 4-methyldiphenylmethylamine resin (MBHA) with trifluoroacetic acid (TFA) for 1 minute, twice, dissolve the Boc-protected amino acid in 0.5M HBTU (the solvent is DMF), and react at room temperature for 20 minutes. Wash with DMF, remove Boc protection with TFA, and connect all amino acids sequentially according to the amino acid sequence. After the reaction, wash the resin, remove the protective group with TFA, and dry it in vacuum. Then put it into a peptide cutting tube, add an appropriate amount of P-cresol, then pass it into HF, stir and react in an ice bath for 1 hour; after the reaction, remove the HF in the tube under reduced pressure. , the residue was precipitated with an appropriate amount of glacial ether, filtered to obtain the precipitate and washed with glacial ether; the precipitate was re-dissolved with TFA, and filtered to obtain the filtrate; ) (se...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com