Amikacin semi-antigen, and preparation method and application thereof
A technology of amikacin and hapten, which is applied in the field of hapten and its preparation, can solve the problems of complex and expensive chromatography, unsuitability for screening, cumbersome processing, etc., and achieve accurate detection method, good affinity, and detection low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Embodiment 1: Synthesis and identification of amikacin hapten (synthetic route such as figure 1 )
[0019] The mixture of 0.59 g amikacin, 0.18 g p-carboxybenzaldehyde and 1 ml pyridine in 20 ml DMSO was stirred and reacted at 60°C for 20 hours, the solvent was evaporated, purified by column chromatography in ethanol-water system The condensation product of amikacin and p-carboxybenzaldehyde was obtained by medium recrystallization with a yield of 70%.
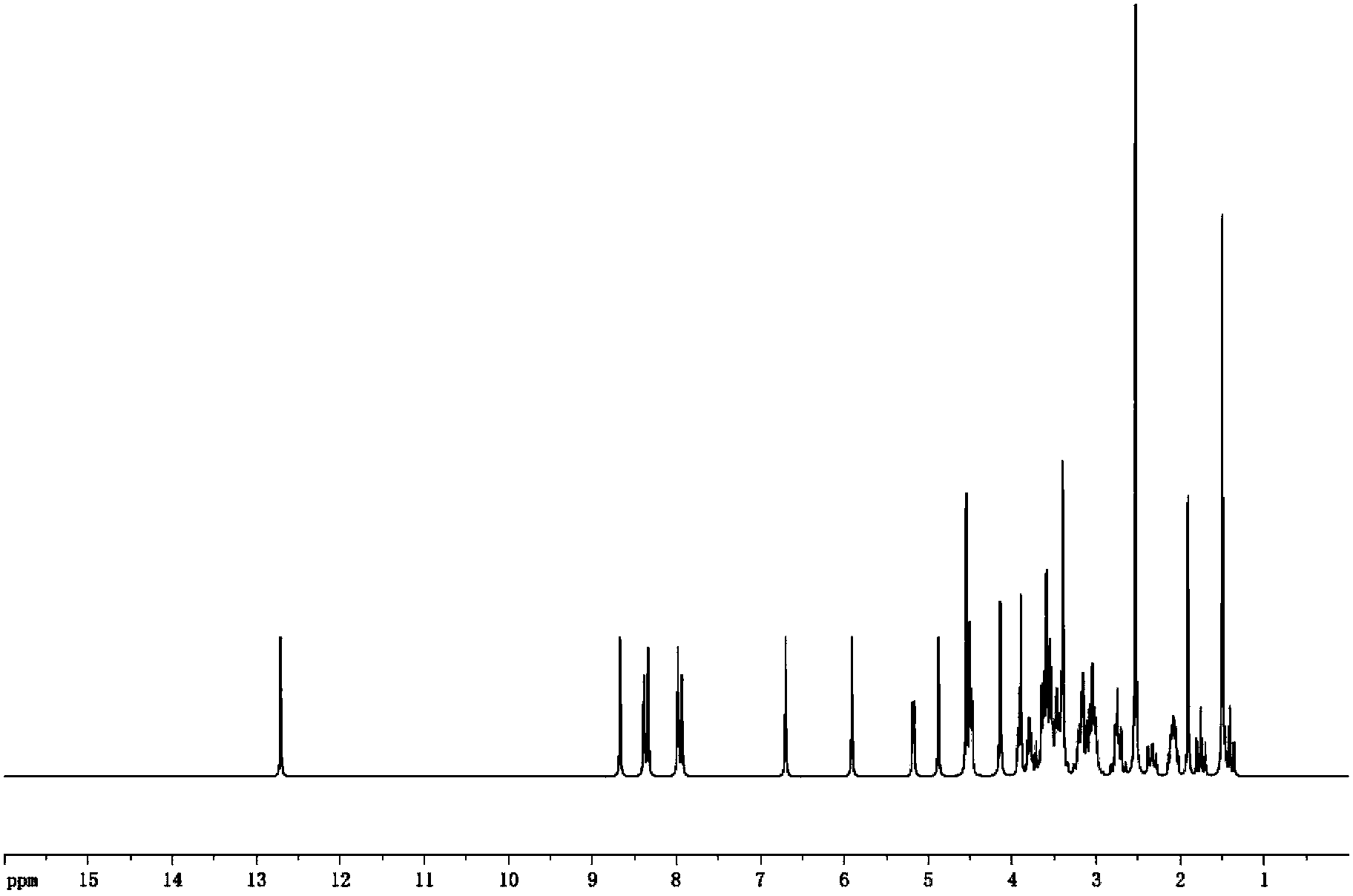
[0020] Get above-mentioned product and measure through proton nuclear magnetic resonance spectrum, such as figure 2 As shown, the nuclear magnetic spectrum shows that: the newly added carboxyl signal peak at about 12.7ppm and the aromatic ring signal peak between 8-9ppm in the spectrum indicate that the hapten is successfully synthesized.
Embodiment 2
[0021] Embodiment 2: Amikacin antigen
[0022] The amikacin hapten is coupled with the carrier protein to obtain the amikacin antigen.
[0023] 1. Immunogen preparation - synthesis of amikacin hapten-bovine serum albumin (BSA) conjugate
[0024] Dissolve 15 mg of amikacin hapten in 1.5 ml of water to obtain solution I; take 10 μl of 50% glutaraldehyde (GA) and add it to solution I, stir and react at room temperature for 18 hours to obtain solution II; take 60 mg of BSA in 4.5 ml Dilute with water and add to solution II; add 24mg NaBH after reacting overnight 4 React for 3 hours; dialyze with triple distilled water for 48 hours to obtain the immunogen.
[0025] 2. Coating source preparation - synthesis of amikacin hapten-ovalbumin (OVA) conjugate
[0026] Dissolve 50mg of carbodiimide (EDC) in 2ml of water to obtain solution III; dissolve 18mg of amikacin hapten in 2ml of water to obtain solution IV; dissolve 30mg of OVA in 1ml of 0.01mol / L PBS (pH =8.0) solution to obtain ...
Embodiment 3
[0029] Embodiment 3: Amikacin monoclonal antibody
[0030] 1. Preparation of Amikacin Monoclonal Antibody
[0031] Animal immunization: Inject the immunogen into the body of Balb / c mice with an immunization dose of 150 μg / mouse to make them produce polyclonal antibodies.
[0032] Cell fusion and cloning: After the measurement result of mouse serum was higher, the splenocytes were taken and fused with SP2 / 0 myeloma cells at a ratio of 8:1, and the cell supernatant was measured by indirect competitive ELISA, and the positive wells were screened. Use the limiting dilution method to clone the positive wells until hybridoma cell lines secreting monoclonal antibodies are obtained. It is found that the titer of one of the hybridoma cell lines is significantly higher than that of the other hybridoma cell lines, and the amikacin monoclonal antibody is hybridized. The tumor cell line was named D-4-3, and the cell line was preserved on May 21, 2012 in the General Microbiology Center of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com