Method for producing patch, and patch
A manufacturing method and a technology for an adhesive, which are applied in the directions of non-active ingredients medical preparations, medical preparations containing active ingredients, pharmaceutical formulas, etc., can solve the problems of coloration of the adhesive layer, poor storage stability, etc., and reduce the Uneven skin permeability and excellent skin permeability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
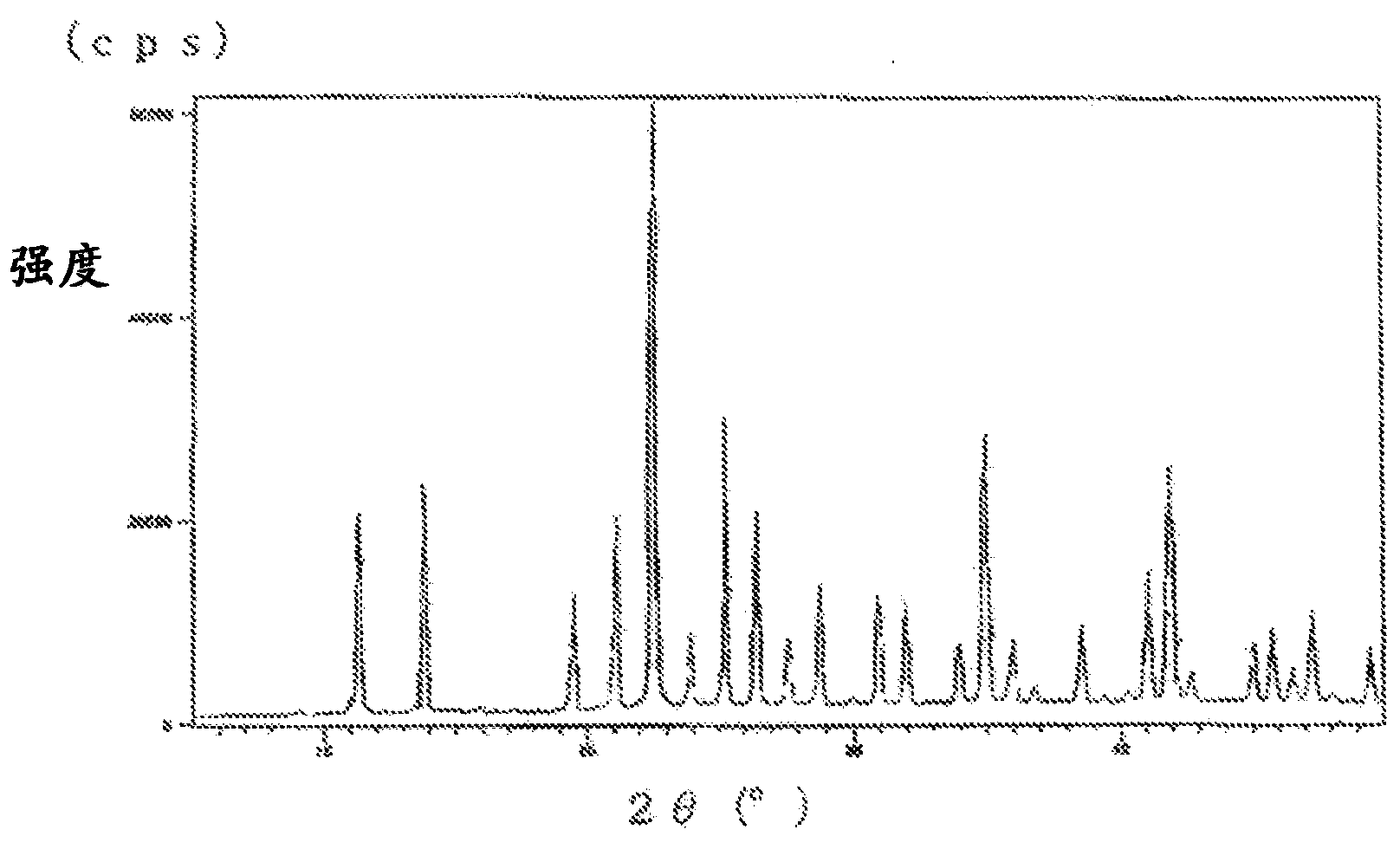
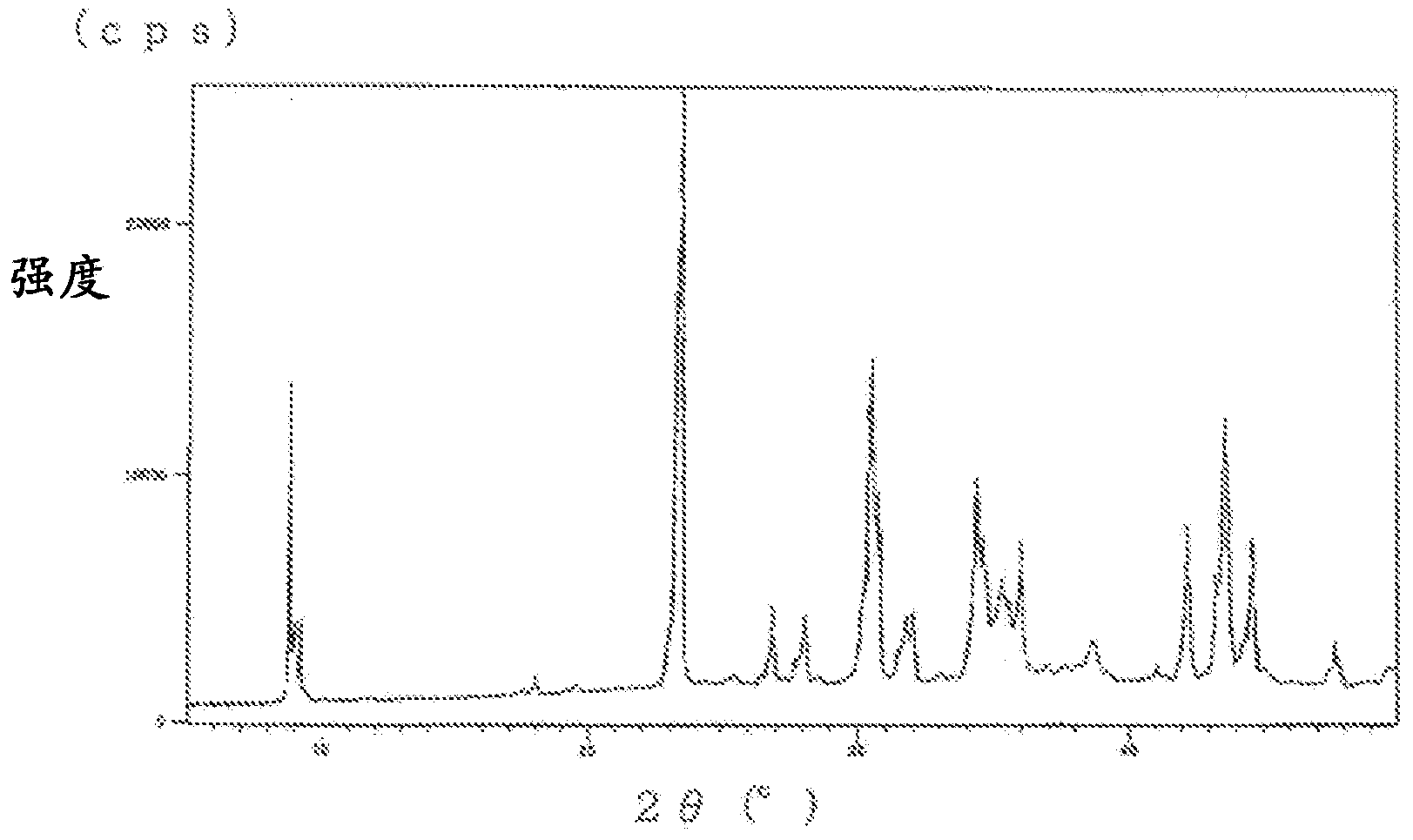
Image
Examples
Embodiment 1
[0120] First, 10.0 parts by mass of sodium diacetate, 5.0 parts by mass of emedastine fumarate, 18.0 parts by mass of styrene-isoprene-styrene block copolymer (SIS), 5.0 parts by mass of methacrylate copolymer (trade name: Eudragit, manufactured by Romfarma), 8.0 parts by mass of polyisobutylene (PIB), 39.0 parts by mass of petroleum-based tackifying resin (trade name: Alcon, manufactured by Arakawa Chemical Industry Co., Ltd.), 5.0 parts by mass of sucrose fatty acid ester, 5.0 parts by mass of sorbitan trioleate, and 5.0 parts by mass of diisopropanolamine are mixed in toluene and stirred by a propeller mixer to obtain a uniform adhesive Layer composition (concentration of non-volatile components: 50% by mass). Next, the pressure-sensitive adhesive layer composition was applied on one side of a release liner layer made of polyethylene terephthalate having a thickness of 75 μm so that the thickness after drying became 100 μm, and dried at 60° C. 20 minutes to form an adhesiv...
Embodiment 2~4
[0122] A patch was obtained in the same manner as in Example 1 except that the composition of the adhesive layer composition was the composition shown in Table 1. In addition, in the adhesive layer of the patch obtained, the molar ratio of emedastine fumarate to sodium diacetate (the number of moles of emedastine fumarate: the molar number of sodium diacetate) Number) is 1:7.5 in embodiment 2, is 1:6 in embodiment 3. In addition, the molar ratio of spatriptyline maleate to sodium diacetate in Example 4 (the number of moles of spatriptyline maleate:the number of moles of sodium diacetate) was 1:10.5.
Embodiment 5
[0127] (Example 5, Comparative Example 5)
[0128] Patch preparations were obtained in the same manner as in Example 1, except that the composition of the adhesive layer composition was the composition shown in Table 2 below. In addition, in the adhesive layer of the patch obtained in Example 5, the molar ratio of oxybutynin hydrochloride to sodium diacetate (the number of moles of oxybutynin hydrochloride:the number of moles of sodium diacetate) was 1 :5.
[0129] [Table 2]
[0130] Composition (parts by mass)
Example 5
Comparative Example 5
Sodium diacetate
18.0
-
-
9.0
-
2.5
20.0
10.5
Petroleum-based tackifying resin
34.7
32.0
SIS
17.3
18.0
PIB
-
13.0
10.0
15.0
total
100.0
100.0
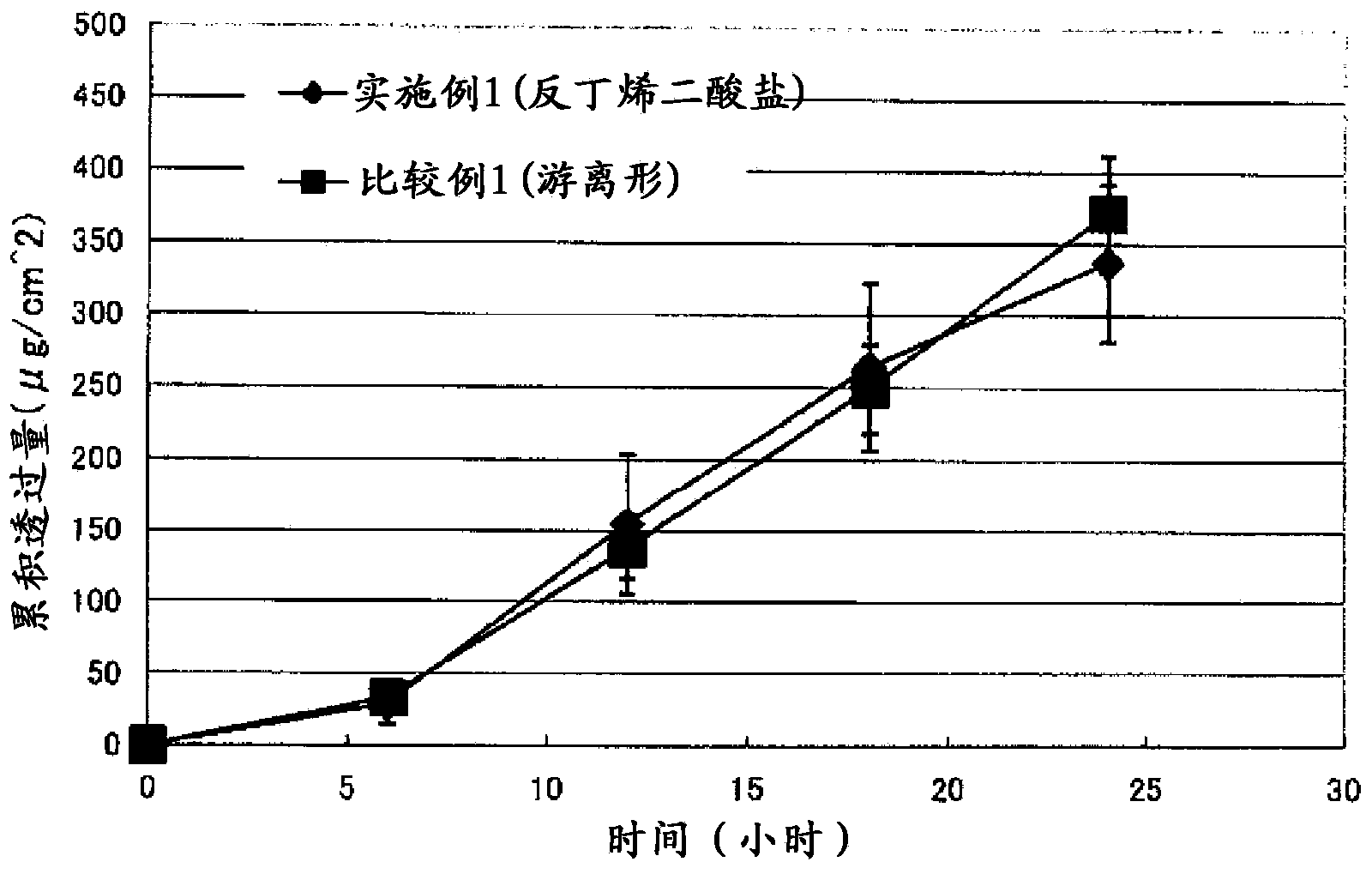
[0131] The skin penetration test was performed on the patches obtaine...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com