Fullerene single catechol derivative intermediate, preparation method thereof and purposes thereof
A technology of fullerene monocatechol and intermediates, which is applied in the field of preparation of polymer materials, can solve the problem of short-term detoxification function, and achieve the effect of broadening performance and function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
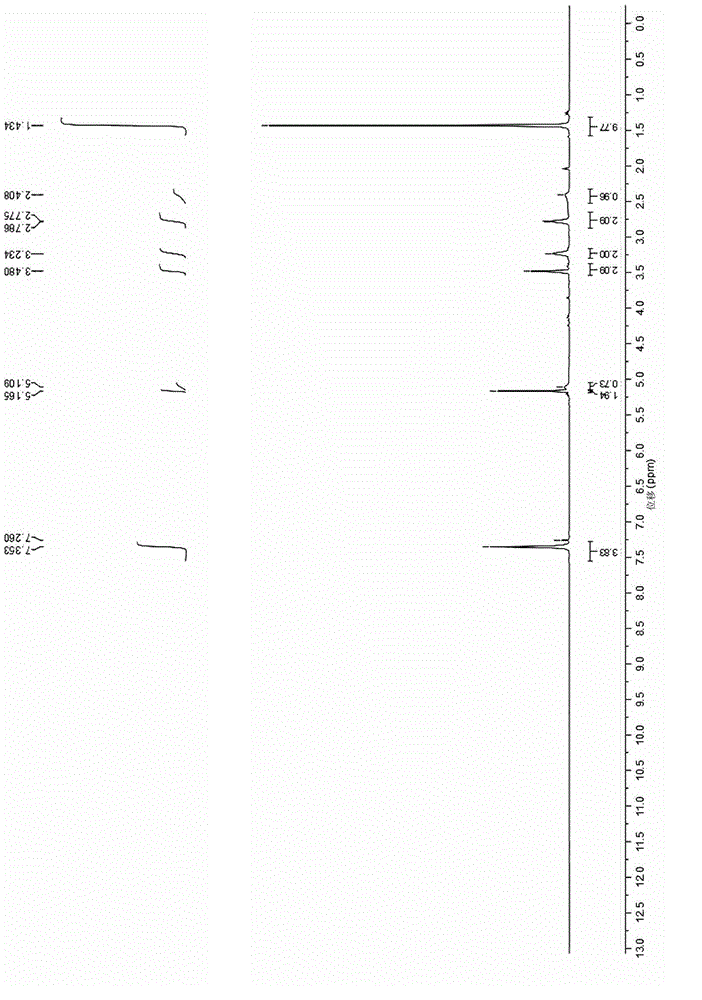
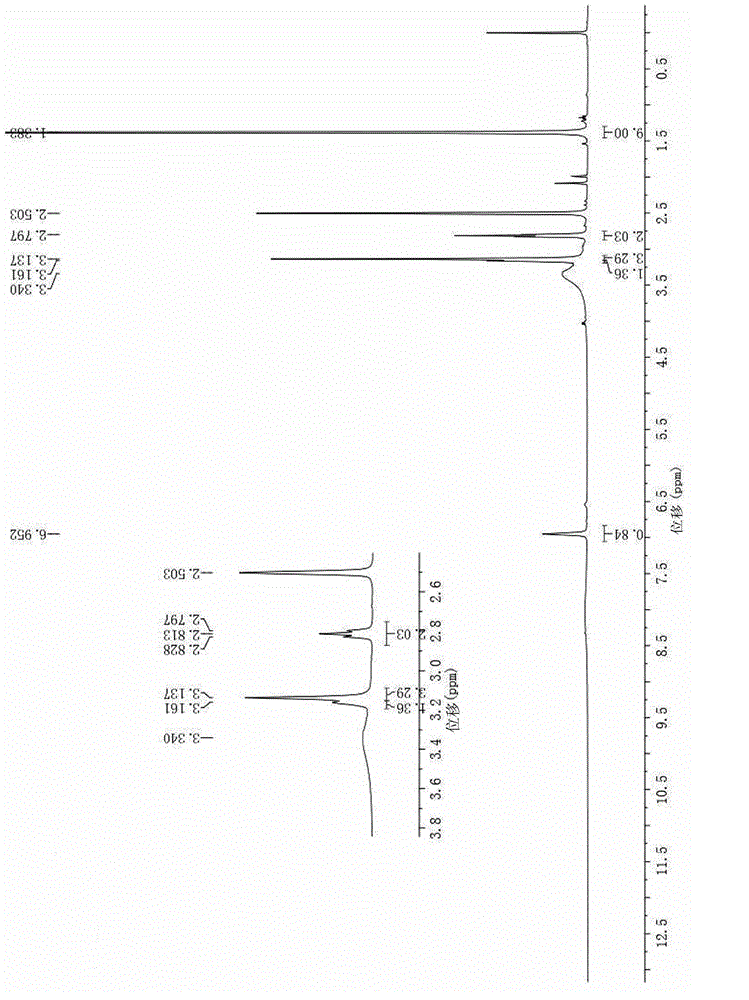
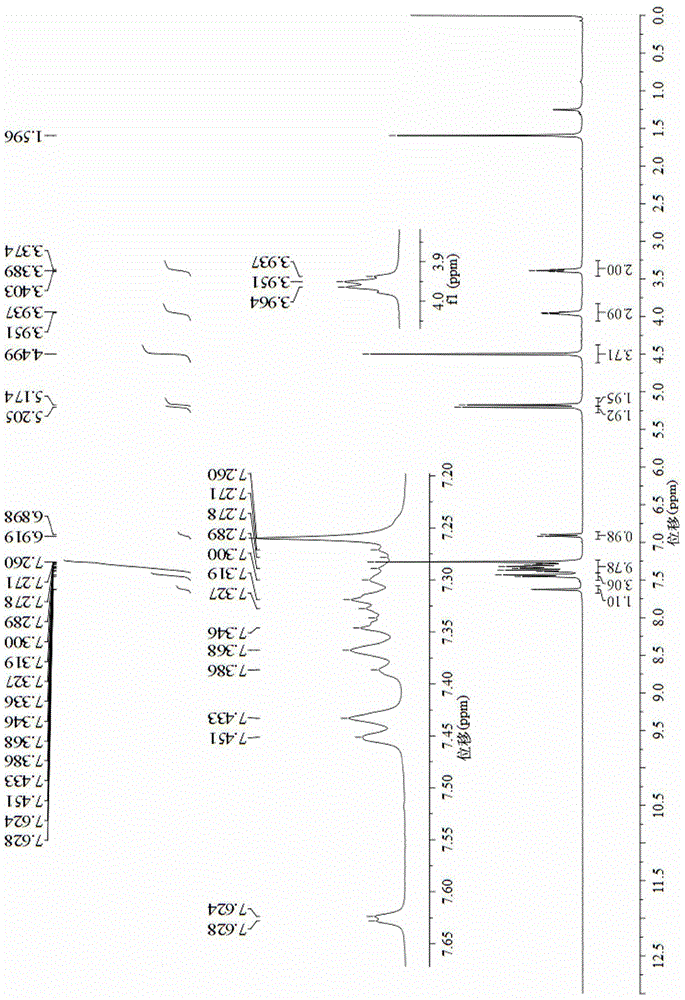
Image
Examples
Embodiment 1
[0048] 1) Preparation of N-Boc-ethylenediamine
[0049] Add 4.5 parts of ethylenediamine and 40 parts of dichloromethane into a reaction flask equipped with a stirrer, and vigorously stir at room temperature. Slowly add Boc to the reaction flask through a constant pressure dropping funnel 2 O12 parts, 40 parts of dichloromethane solution, the whole dropwise addition process continued for 8 to 10 hours, continued to stir for 18 to 22 hours, then removed the solvent under reduced pressure, concentrated to 100 to 110 parts, poured 10 parts of sodium carbonate solution (aq. 20%), separated, the aqueous phase was extracted three times with dichloromethane, each time with 10 parts of dichloromethane, combined with the organic phase, dried with anhydrous magnesium sulfate, filtered, and the solvent was removed under reduced pressure to obtain a colorless 9.14 parts of mono-Boc-protected ethylenediamine product, yield 98%, without further purification, the product was directly used i...
Embodiment 2
[0063] Compounds (1) to (4) were prepared according to the preparation methods 1) to 4) in Example 1;
[0064] 5) Preparation of Intermediate IV
[0065] Suspend 0.02 parts of Intermediate III in 0.6 parts of dichloromethane, add 0.04 parts of trifluoroacetic acid, stir at room temperature, and monitor the progress of the reaction by TLC (developer: petroleum ether: ethyl acetate = 2:1). The reaction time is 10 h, and the reaction After the end, add 2mol·L -1 NaOH solution to neutralize to a pH value of 13.5, filter with suction, and wash the filter cake three times with water. Obtained 0.15 parts of solid, yield 85.3%;
[0066] 6) Preparation of target objects
[0067] Add 0.6 parts of 3,4-dihydroxybenzoic acid into a reaction flask with a stirrer, then add 1 part of dimethylformamide (DMF) to dissolve, and slowly add 2.7 parts of K to the solution under stirring. 2 CO 3 , then add 3.5 parts of benzyl bromide dropwise under stirring at room temperature, stir overnight af...
Embodiment 3
[0072] Compounds (1) to (4) were prepared according to the preparation methods 1) to 4) in Example 1;
[0073] 5) Preparation of Intermediate IV
[0074] Suspend 0.02 parts of intermediate III in 0.8 parts of dichloromethane, add 0.06 parts of trifluoroacetic acid, stir at room temperature, and monitor the reaction progress by TLC (developing solvent: petroleum ether: ethyl acetate = 2:1), the reaction time is 10.5h, After the reaction, add 2mol L -1 The NaOH solution was neutralized to a pH value of 14, suction filtered, and the filter cake was washed three times with water to obtain 0.1 part of solid, yield: 87%;
[0075] 6) Preparation of target objects
[0076] Add 6 parts of 3,4-dihydroxybenzoic acid into a reaction flask with a stirrer, then add 8 parts of DMF to dissolve, and slowly add 28 parts of K to the solution under stirring. 2 CO 3 , then add 35 parts of benzyl bromide dropwise under stirring at room temperature, stir overnight after the dropwise addition is ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com