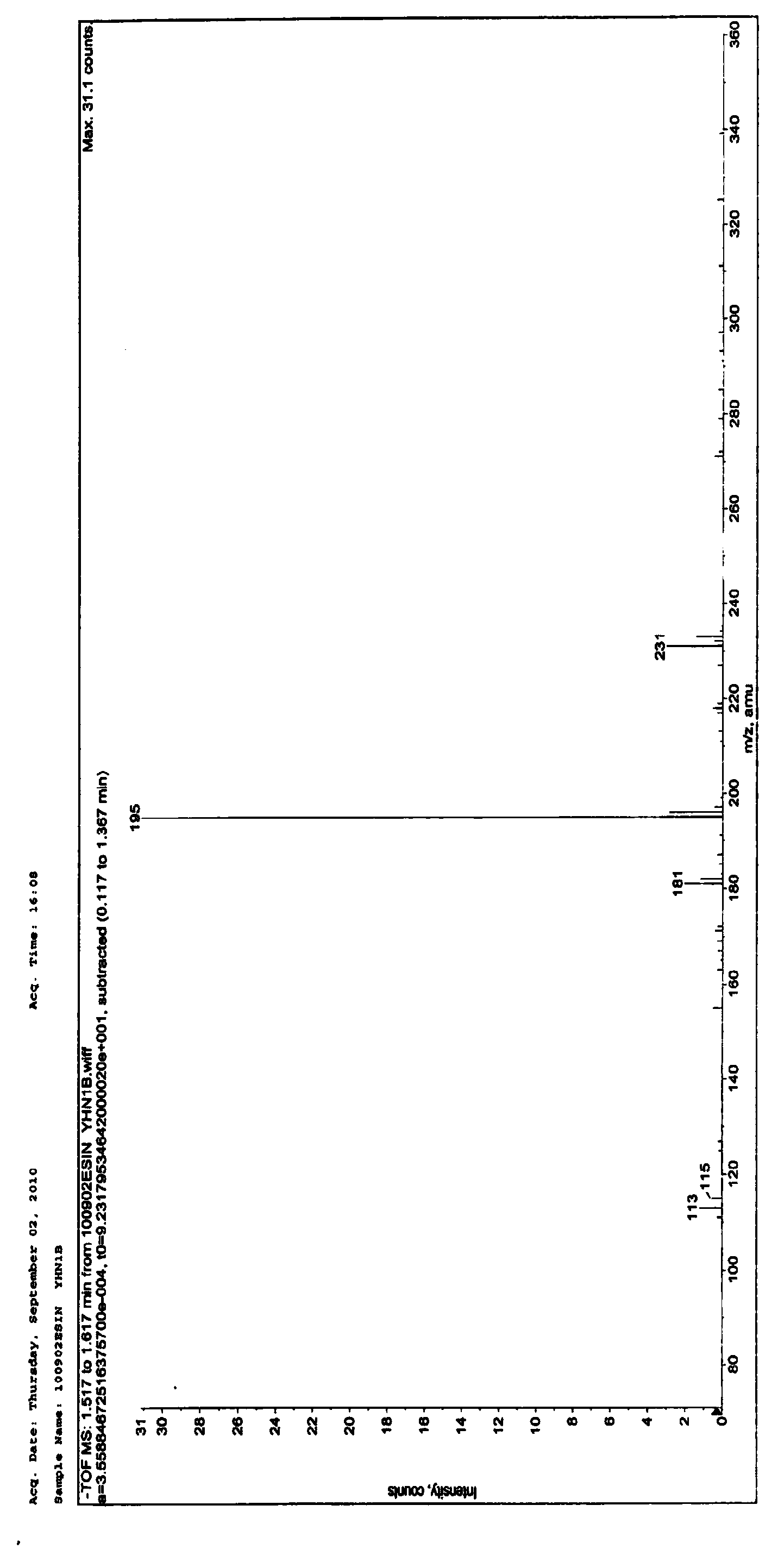
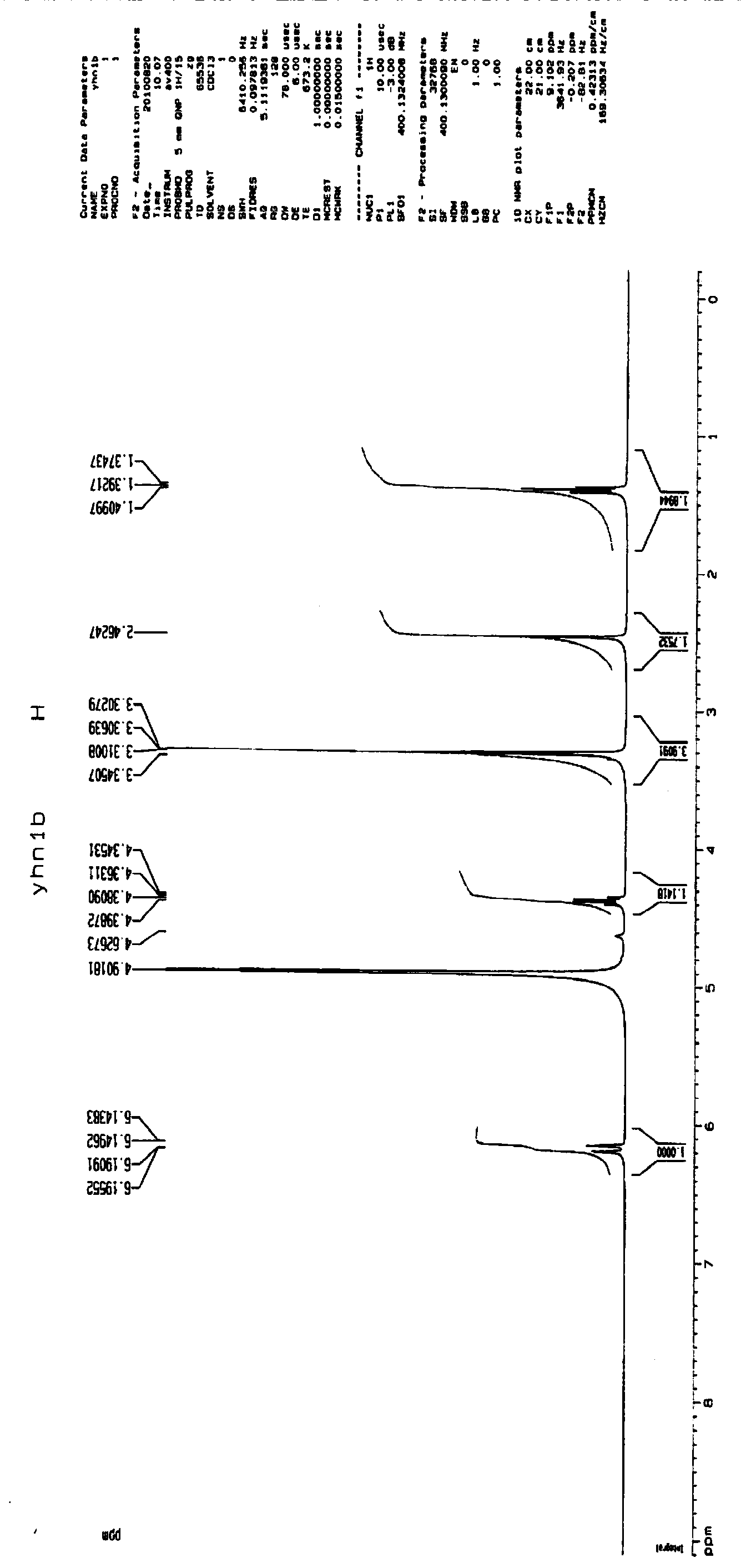
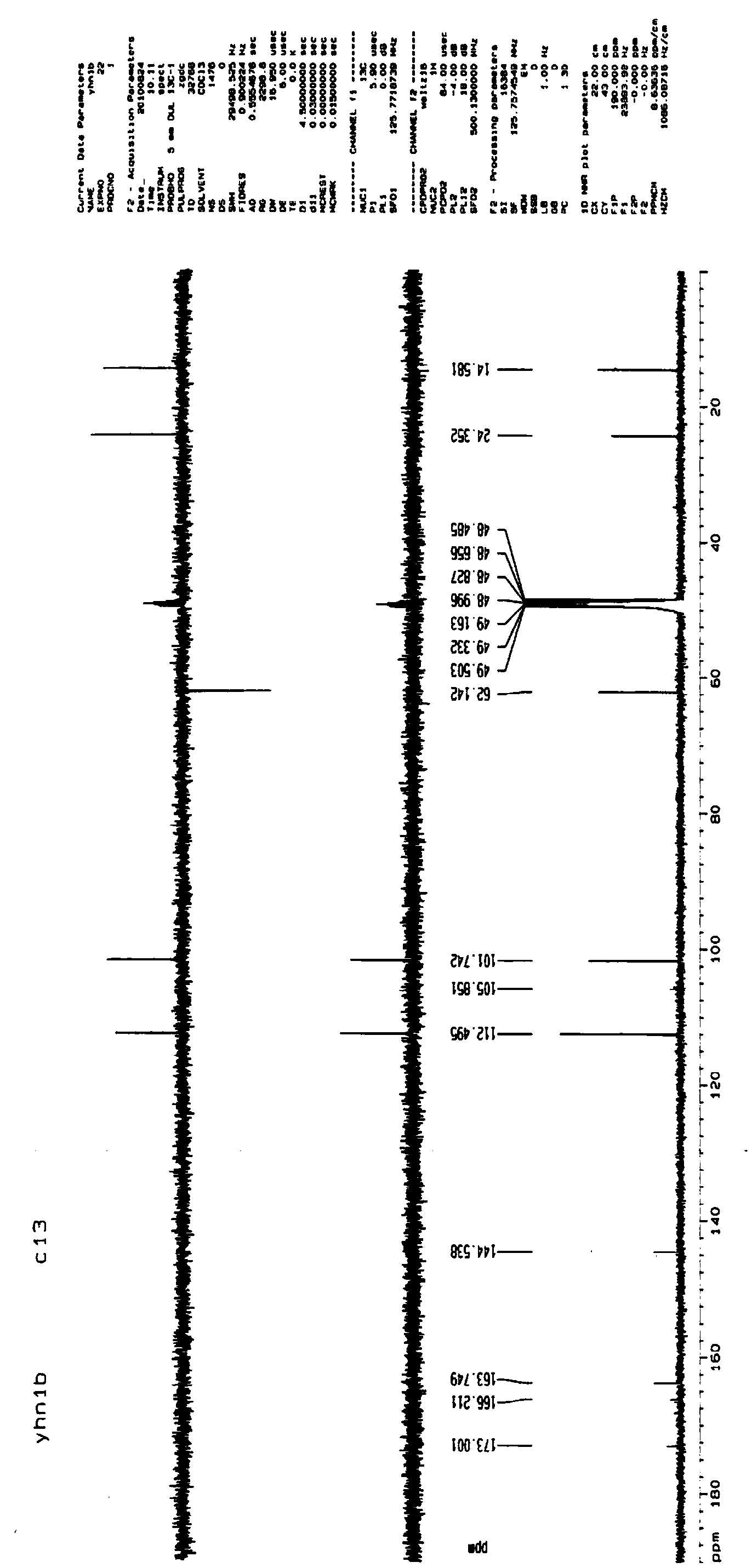
4-oxethyl-2-hydroxyl-6-methyl benzoic acid as well as medicinal composition and application thereof
A technology of methyl benzoic acid and ethoxy, which is applied in the field of chemical medicine, can solve the problems of high toxicity and side effects of drugs, drug resistance of cancer cells, and uncertain curative effect, so as to inhibit the proliferation of cancer cells and regulate the cycle and differentiation of cancer cells , the effect of promoting apoptosis of cancer cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Example 1: Synthesis and structural identification of the compound 4-ethoxy-2-hydroxy-6-methylbenzoic acid (4-ethoxy-2-hydroxy-6-methylbenzoic acid) represented by formula (1)
[0049] 1. Preparation method
[0050] Phase Transfer Catalytic Synthesis and Column Chromatographic Separation
[0051] Using phase transfer catalysis, in the presence of sodium hydroxide, 2,4-dihydroxy-6-methylbenzoic acid and diethyl sulfate (molar ratio is 0.2:0.18), in the catalyst HA-1 (dioctadecane Base methylamine benzyl quaternary ammonium chloride) under the action of 60-65 ℃ for 3h. After the reaction is completed, cool to 25°C, pour into ice water, adjust the pH value to neutral with dilute hydrochloric acid, separate the organic layer; extract the water layer with toluene twice, combine the organic layer; wash the organic layer three times with anhydrous sulfuric acid Sodium dry. The solvent toluene was distilled off under reduced pressure, and the product was separated and purifi...
Embodiment 2
[0056] Embodiment 2: The biological experiment result of formula (1) compound 4-ethoxyl-2-hydroxyl-6-methylbenzoic acid
[0057] The following are some specific experimental examples to illustrate the formula (1) compound 4-ethoxy-2-hydroxy-6-methylbenzoic acid of the present invention (indicated by D6B2 or D in the following charts, the same below; D1 is 200 μM, D2 150 μM for D3, 100 μM for D3, 50 μM for D4, and 25 μM for D5) have significant and selective anti-tumor effects, combined anti-tumor effects with clinical anti-tumor drugs, as well as their toxicological safety and anti-tumor mechanism.
[0058] 1. In vitro anticancer effect of 4-ethoxy-2-hydroxy-6-methylbenzoic acid:
[0059] Test method: The compound 4-ethoxy-2-hydroxy-6-methylbenzoic acid of formula (1) was dissolved in DMSO, and the anticancer test was carried out in vitro according to the final concentration of 25, 50, 100, 150 and 200 μM. Human or animal tumor cells in logarithmic growth phase were collected...
Embodiment 3
[0110] Formula (1) compound 4-ethoxy-2-hydroxy-6-methylbenzoic acid and sodium bicarbonate are neutralized under heating conditions, then adjust the pH value to 7.0-7.5 with soda ash solution, heat, evaporate, Crystallization forms the sodium salt of the compound of formula (1), Sodium4-ethoxy-2-hydroxy-6-methylbenzoate, which is soluble in water. The compounds of formula (1) can also form salts with other basic ions or groups.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com