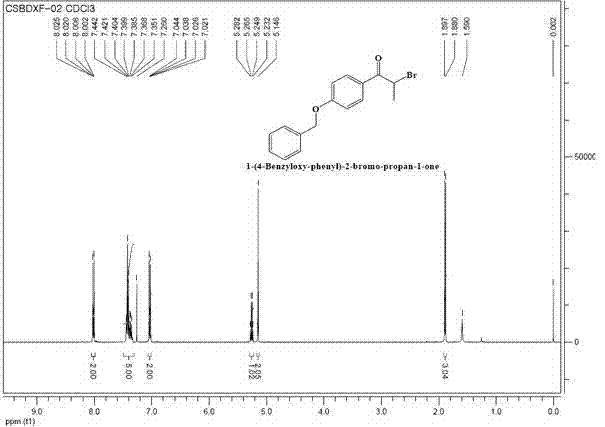
Preparation method for intermediate (namely 1-(4-benzyloxy-phenyl)-2-bromo-propan-1-one) for bazedoxifene acetate
A technology of bazedoxifene acetate and benzyloxy is applied in the field of preparation of bazedoxifene acetate intermediate 1--2-bromopropyl-1-one, which can solve the problem of unsuitability for industrial production and poor bromine selectivity , the reaction is difficult to operate and other problems, to achieve the effect of improving selectivity, high selectivity and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] (1) Add 24g of 1-(4-benzyloxy-phenyl)-2-propyl-1-one and 45g of CuBr to a 1L three-necked flask 2 , 250 mL ethyl acetate, 250 mL chloroform, heated to reflux, tracked and monitored by TLC plate until the reaction of the main raw material (1-(4-benzyloxy-phenyl)-2-propyl-1-one) was complete, stopped heating and cooled to room temperature (25°C), and the reaction was completed to obtain a reaction solution.
[0019] (2) The reaction solution was suction filtered through a sand core funnel, the filtrate was evaporated to remove the solvent, and 37.6g of a crude brown oil was obtained. Add 250mL of absolute ethanol to the crude product, and the system was turbid and heated to reflux for 0.5h. Stop heating and cool to -5-0 The product was crystallized at ℃, washed with suction, and the filtrate was concentrated and then refluxed and crystallized again to obtain a total of 31.2 g of the product as a white solid (98% yield).
Embodiment 2
[0021] (1) Add 24g of 1-(4-benzyloxy-phenyl)-2-propyl-1-one, 24g of CuBr to a 1L three-necked flask 2 , 200 mL of a mixture of dichloromethane and 1,2-dichloroethane (the volume ratio of the two is 1:1), 200 mL of methyl formate, heating to reflux, TLC plate tracking monitoring to the main raw material (1-(4-benzyl Oxygen-phenyl)-2-propyl-1-one) reaction is complete, stop heating, cool to room temperature (25°C), and the reaction is completed to obtain the reaction solution.
[0022] (2) The reaction solution was suction filtered through a sand core funnel, the filtrate was evaporated to remove the solvent, and 36.7g of a crude brown oil was obtained. Add 230mL of ethyl acetate to the crude product, and the system was turbid and heated to reflux for 1h. Stop heating and cool to -15--10 The product was crystallized at ℃, washed with suction, and the filtrate was concentrated and then refluxed and crystallized again to obtain a total of 30.3 g of the product as a white solid (yi...
Embodiment 3
[0024] (1) Add 24g of 1-(4-benzyloxy-phenyl)-2-propyl-1-one, 240g of CuBr to a 1L three-necked flask 2 , 230 mL of a mixture of methyl formate, ethyl formate and methyl acetate (the volume ratio of the three is 1:2:1), 200 mL of DMF, heated to reflux, followed by TLC plate monitoring to the main raw material (1-(4-benzyl Oxygen-phenyl)-2-propyl-1-one) reaction is complete, stop heating, cool to room temperature (25°C), and the reaction is completed to obtain the reaction solution.
[0025] (2) The reaction solution was filtered through a sand core funnel, the filtrate was evaporated to remove the solvent, and 37.0g of the crude brown oil was obtained. Add 230mL of DMF to the crude product, and the system was turbid and heated to reflux for 2 hours. Stop heating and cool to -10--5°C to make the product Crystals were precipitated, filtered and washed with suction, and the filtrate was concentrated and refluxed and crystallized again to obtain a total of 30.9 g of the product as ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com