Palladium-free chemical copper-plating method on graphite nanosheet surface
A technology of nano-graphite microflakes and electroless copper plating, which is applied in liquid chemical plating, metal material coating process, coating, etc., can solve the problems of expensive palladium catalyst, low deposition rate of copper plating, low stability of plating solution, etc. problems, to achieve good mechanical properties, low equipment requirements, and excellent electrical conductivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
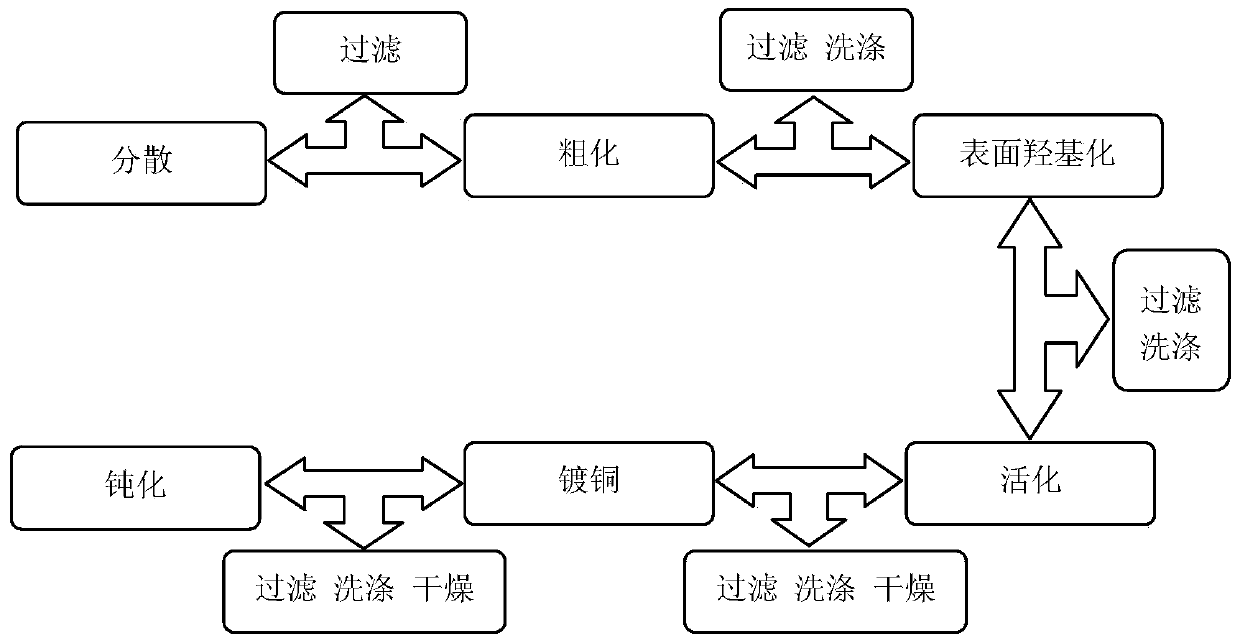
[0035] Step 1: Dispersion treatment: Weigh 0.1g of nano-graphite microflakes and ultrasonically disperse them in 50ml of absolute ethanol for 15 minutes, then filter to obtain dispersed nano-graphite microflakes;
[0036] The second step: coarsening treatment: put the nano-graphite microflakes obtained in the first step into 100ml of 20g / L NaOH solution, sonicate at 35°C for 30 minutes, then filter, wash with deionized water until the filtrate is neutral sex;
[0037] The third step: surface hydroxylation treatment: putting the nano-graphite microflakes after the roughening treatment in the second step into 50ml concentration of hydrogen peroxide and 60g / L ammonia solution, and continuously stirring, the reaction temperature is 85°C, the reaction time is 50 minutes, then filter, wash with deionized water until the filtrate is neutral, and obtain nano-graphite microflakes with a large number of hydroxyl groups on the surface;
[0038] The fourth step: activation treatment: wei...
Embodiment 2
[0046] Step 1: Dispersion treatment: Weigh 0.1g of nano-graphite microflakes and ultrasonically disperse them in 50ml of absolute ethanol for 15 minutes, then filter to obtain dispersed nano-graphite microflakes;
[0047] The second step: coarsening treatment: put the nano-graphite microflakes obtained in the first step into 100ml of 20g / L NaOH solution, sonicate at 35°C for 30 minutes, then filter, wash with deionized water until the filtrate is neutral sex;
[0048] The third step: surface hydroxylation treatment: putting the nano-graphite flakes after the roughening treatment in the second step into 50ml concentration of hydrogen peroxide and 80g / L ammonia solution, and continuously stirring, the reaction temperature is 85°C, the reaction time is 50 minutes, then filter, wash with deionized water until the filtrate is neutral, and obtain nano-graphite microflakes with a large number of hydroxyl groups on the surface;
[0049] The fourth step: activation treatment: weigh 0....
Embodiment 3
[0057] Step 1: Dispersion treatment: Weigh 0.2g of nano-graphite microflakes and ultrasonically disperse them in 100ml of absolute ethanol for 20 minutes, then filter to obtain dispersed nano-graphite microflakes;
[0058] The second step: coarsening treatment: put the nano-graphite microflakes obtained in the first step into 200ml of 20g / L NaOH solution, ultrasonicate at 35°C for 35 minutes, then filter, wash with deionized water until the filtrate is neutral sex;
[0059] The third step: surface hydroxylation treatment: putting the nano-graphite flakes after the roughening treatment in the second step into 100ml concentration of hydrogen peroxide and 60g / L ammonia solution, and continuously stirring, the reaction temperature is 85°C, the reaction time is 50 minutes, then filter, wash with deionized water until the filtrate is neutral, and obtain nano-graphite microflakes with a large number of hydroxyl groups on the surface;
[0060] The fourth step: activation treatment: w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com