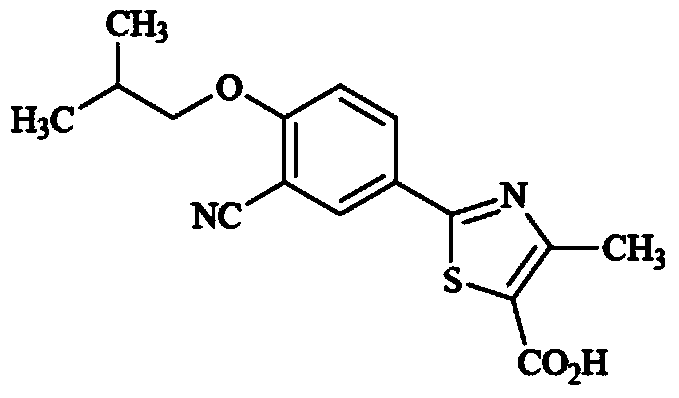
Drug chemical compound for gout
A technology of pharmaceutical compounds, compounds, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0145] Embodiment 1: preparation febuxostat bulk drug
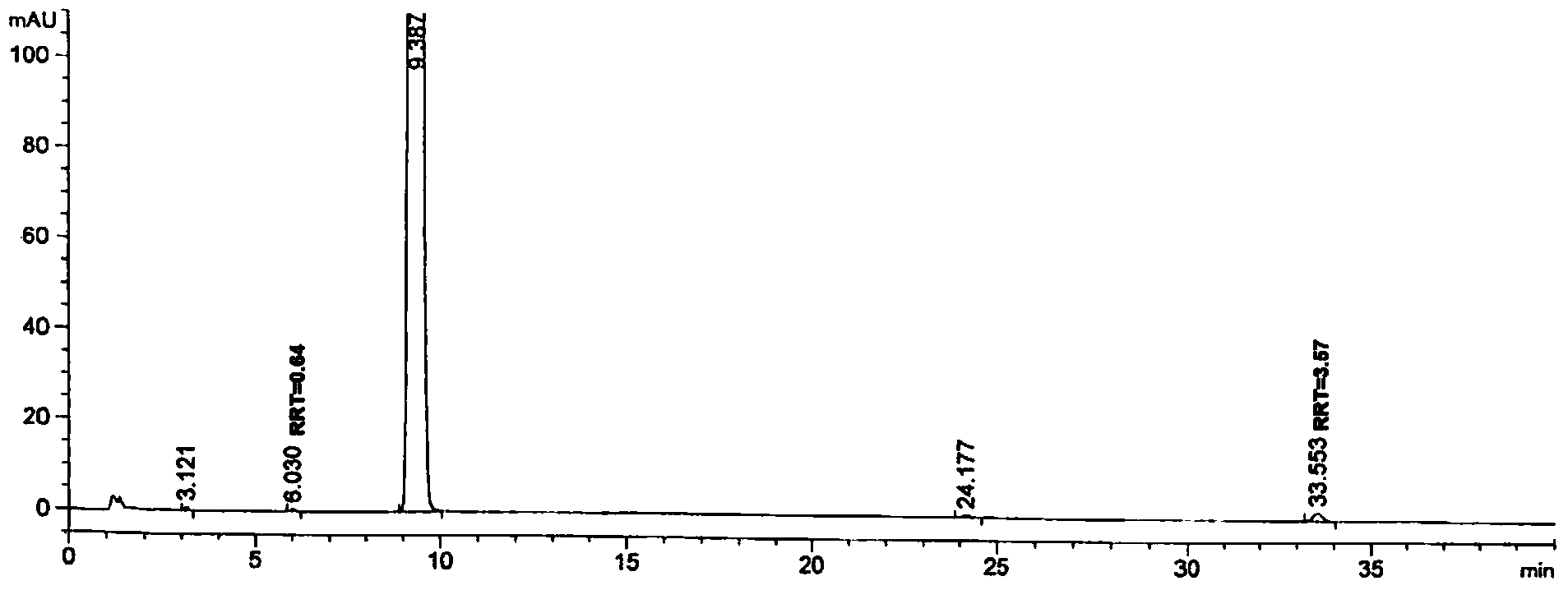
[0146] Step (1): Prepare according to the method described in paragraphs [0022] to [0042] of the specification of Chinese Patent Application No. 200910170029.3 (CN102002016A, Medicom), to obtain the refined febuxostat product. As determined by 【High Performance Liquid Chromatography A】, RRT3.55 content = 0.31%, RRT0.65 content = 0.23%. The chromatographic purity of the product recorded in CN102002016A is greater than 99.5% and less than 0.1% of simple impurities, which is calculated by the "normalization method" different from the method of the present invention, and its detection conditions such as detection wavelength may be different from the present invention, so it is not comparable property; but the RRT3.55 content and the RRT0.65 content of the product of this step (1) are measured by the same assay method as the following product, so their results are comparable.
[0147] Step (2): Refining: Add 1000ml of ethan...
Embodiment 2
[0151] Embodiment 2: preparation febuxostat bulk drug
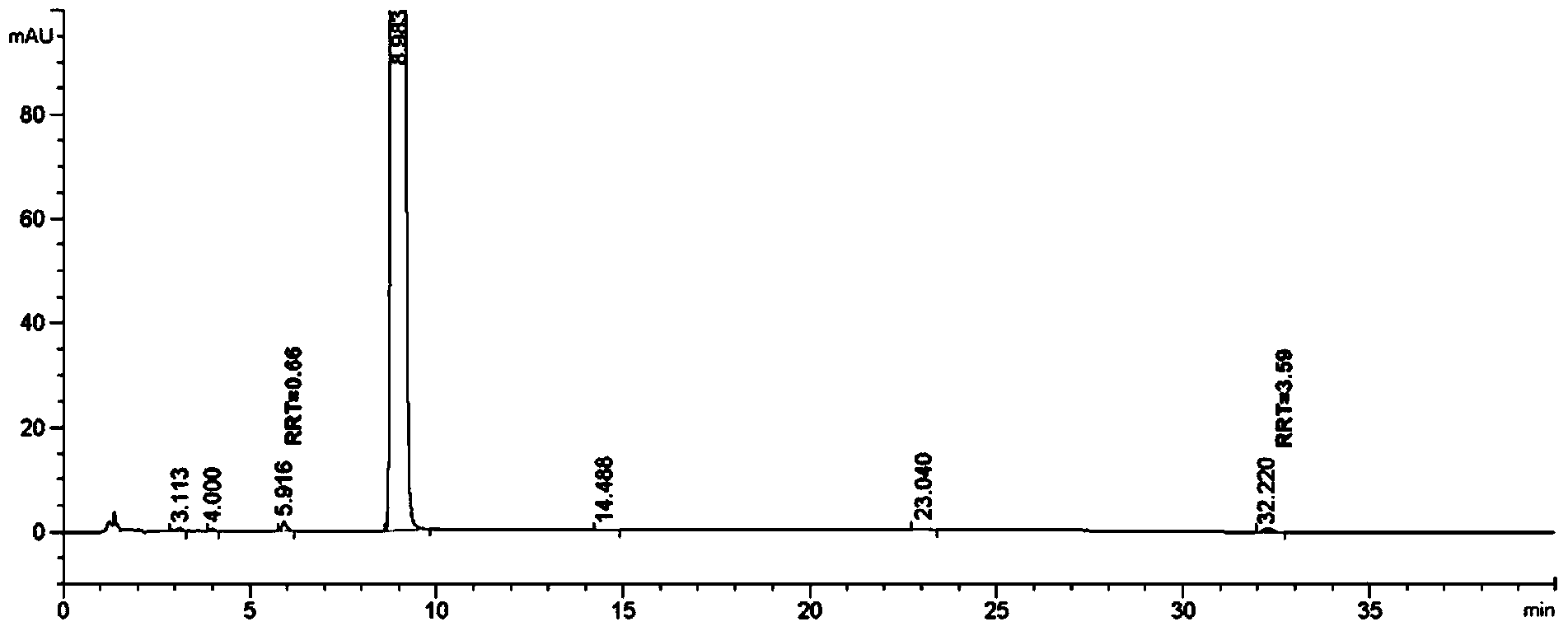
[0152] Add 1000ml of ethanol-acetonitrile-formic acid (100:20:5) mixture to the refining tank in advance, start stirring and add 100g of the product obtained in step (1) of Example 1 of the present invention (solvent: solute = 10 (v / w)) , heating at a temperature 10°C lower than the reflux temperature until all the solids are dissolved, then stop heating, cool down to 20-25°C, continue stirring for 10-12 hours, cool down to 0-10°C, keep stirring for 1-2 hours, Centrifuge to obtain a product that can be used as febuxostat bulk drug (the yield of such refining once is 93.3%). As determined by 【High Performance Liquid Chromatography A】, RRT3.55 content = 0.08%, RRT0.65 content = 0.06%.
Embodiment 3
[0153] Embodiment 3: preparation febuxostat bulk drug
[0154] Add 1000ml of ethanol-acetonitrile-formic acid (100:40:2) mixture to the refining tank in advance, start stirring and add 130g of the product obtained in step (1) of Example 1 of the present invention (solvent: solute = 7.7 (v / w)) , heated at reflux temperature until all the solids were dissolved, then stopped heating, cooled to 20-25°C, continued to stir for 10-12 hours, cooled to 0-5°C, kept stirring for 1-2 hours, and centrifuged to obtain non- The product of Buxostat bulk drug (94.1% of the yield of such refining once). As determined by 【High Performance Liquid Chromatography A】, RRT3.55 content = 0.11%, RRT0.65 content = 0.08%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com