Preparation method of 1-methyl-3,4-binitro pyrromonazole
A technology of dinitropyrazole and methyl, which is applied in the field of preparation of 1-methyl-3,4-dinitropyrazole, can solve the problems of harsh operating conditions, low product yield, environmental pollution, etc., and achieve The raw materials are easy, the synthesis process is simple, and the effect of improving product purity and yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
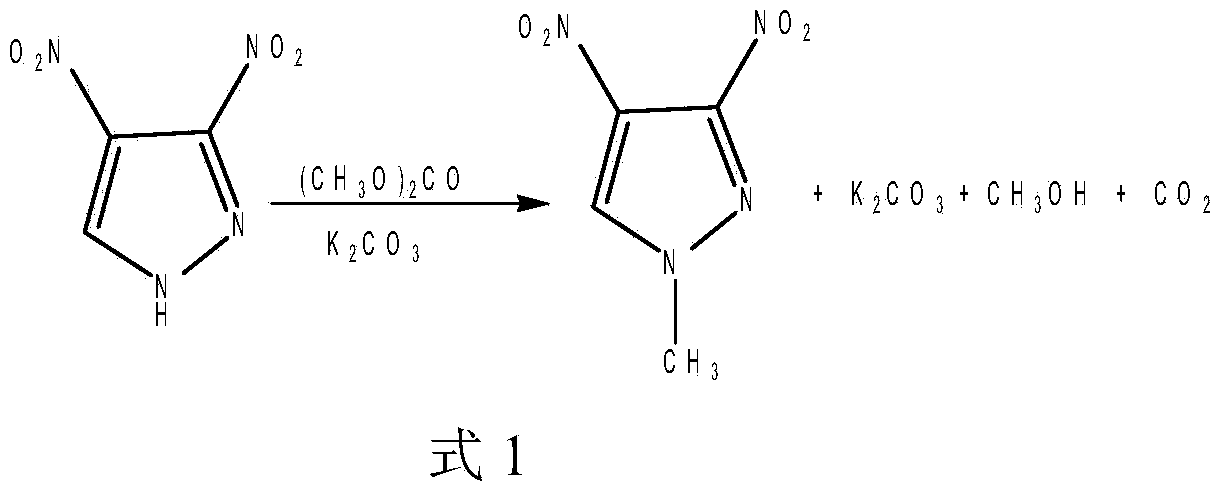
[0016] First, weigh 4.36g of raw materials 3,4-dinitropyrazole, 5.75g of anhydrous potassium carbonate, 10mL of dimethyl carbonate, 41mL of N,N-dimethylformamide, and then add N,N-dimethylformamide Add amide (DMF) into a reaction vessel equipped with a stirrer and a thermometer, place the reaction vessel at a low temperature of 0-5°C, and dry and grind the fine powder of 3,4-dinitropyrazole (DNP) slowly Add in the reaction vessel, after 3,4-dinitropyrazole (DNP) dissolves completely, anhydrous potassium carbonate (K 2 CO 3 ) and dimethyl carbonate (DMC) were added to the reaction solution in the container in sequence. After the addition was complete, stir and keep warm at 60°C for 4 hours, then pour the reaction solution into a container filled with 300mL distilled water, and stir well until the liquid turned dark red. , and after oil droplets appeared, use dichloromethane (CH 2 Cl 2 ) extraction, the organic phases were combined, the solvent was removed, and benzene was re...
Embodiment 2
[0025] First, weigh 4.36g of raw materials 3,4-dinitropyrazole, 5.75g of anhydrous potassium carbonate, 15mL of dimethyl carbonate, 45mL of N,N-dimethylformamide, and then add N,N-dimethylformamide Add amide (DMF) into a reaction vessel equipped with a stirrer and a thermometer, place the reaction vessel at a low temperature of 0-5°C, and slowly add the dried and ground 3,4-dinitropyrazole (DNP) fine powder In the reaction vessel, after 3,4-dinitropyrazole (DNP) is completely dissolved, anhydrous potassium carbonate (K 2 CO 3 ) and dimethyl carbonate (DMC) were sequentially added to the reaction liquid in the container. After the addition was complete, the reaction was stirred and kept at 80°C for 4.5 hours. Red, and after the appearance of oil droplets, dichloromethane (CH 2 Cl 2 ) extraction, the organic phases were combined, the solvent was removed, and benzene was recrystallized to obtain 4.30 g of a light yellow crystal product with a yield of 90%.
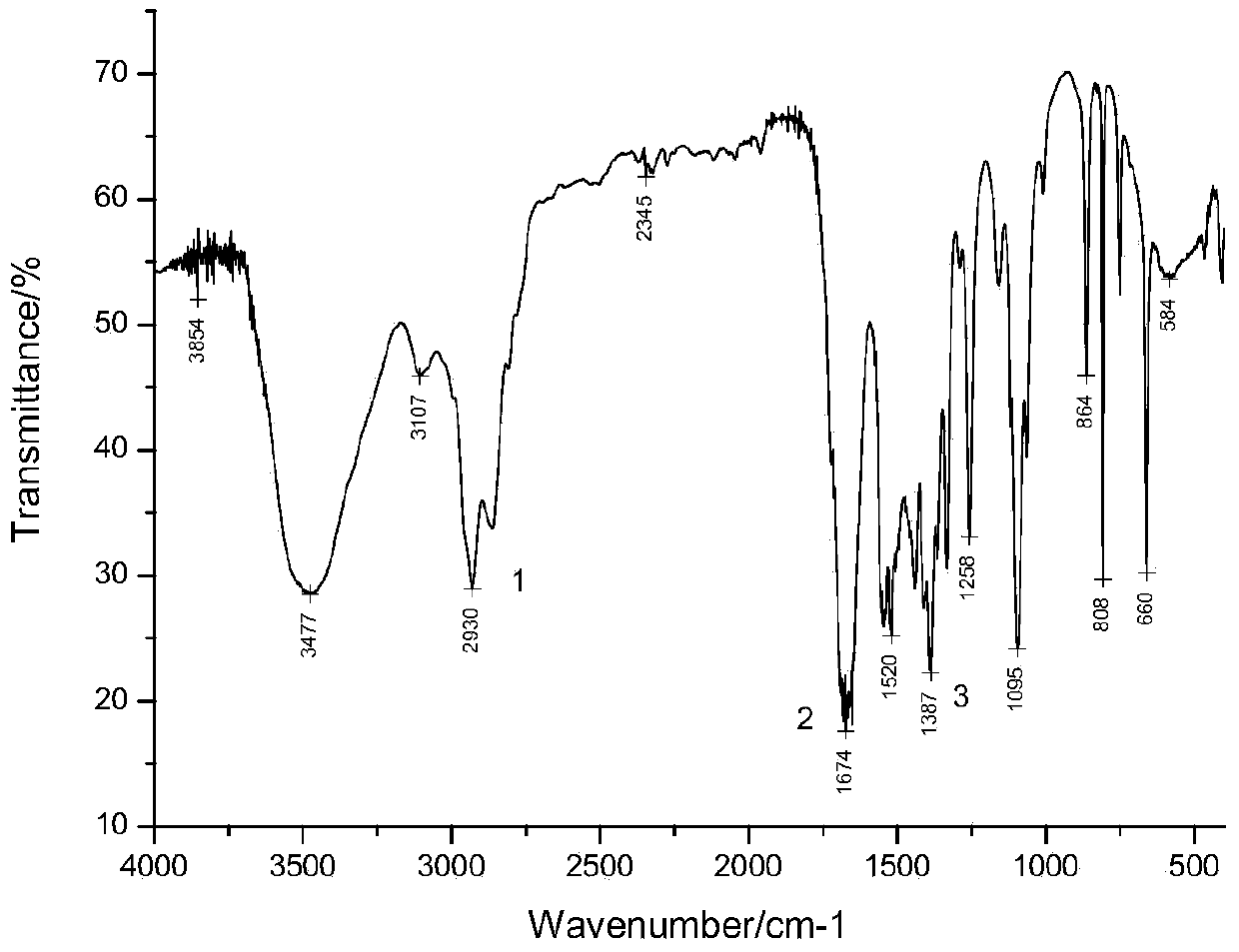
[0026] The synthe...
Embodiment 3
[0033] First, weigh 4.36g of raw materials 3,4-dinitropyrazole, 5.75g of anhydrous potassium carbonate, 20mL of dimethyl carbonate, 47mL of N,N-dimethylformamide, and then add N,N-dimethylformamide Add amide (DMF) into a reaction vessel equipped with a stirrer and a thermometer, place the reaction vessel at a low temperature of 0-5°C, and dry and grind the fine powder of 3,4-dinitropyrazole (DNP) slowly Add in the reaction vessel, after 3,4-dinitropyrazole (DNP) dissolves completely, anhydrous potassium carbonate (K 2 CO 3 ) and dimethyl carbonate (DMC) were added to the reaction solution in the container in turn. After the addition, stirred and kept at 85°C for 7 hours, then poured the reaction solution into a container filled with 300mL of distilled water, and stirred until the liquid was dark red. , and after oil droplets appeared, use dichloromethane (CH 2 Cl 2 ) extraction, the organic phases were combined, the solvent was removed, and benzene was recrystallized to obt...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com