Method for preparing polyethyleneimine modified PLGA (poly(lactic-co-glycolic acid)) loading hollow micro-capsules based on polyethylene glycol and folic acid grafting
A technology of polyethyleneimine and polyethylene glycol, which is applied in the direction of pharmaceutical formulations, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve the problem of the preparation of anticancer drugs that have not been found in PLGA hollow microcapsules To achieve good cytotoxicity, reduce aggregation, and good dispersibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
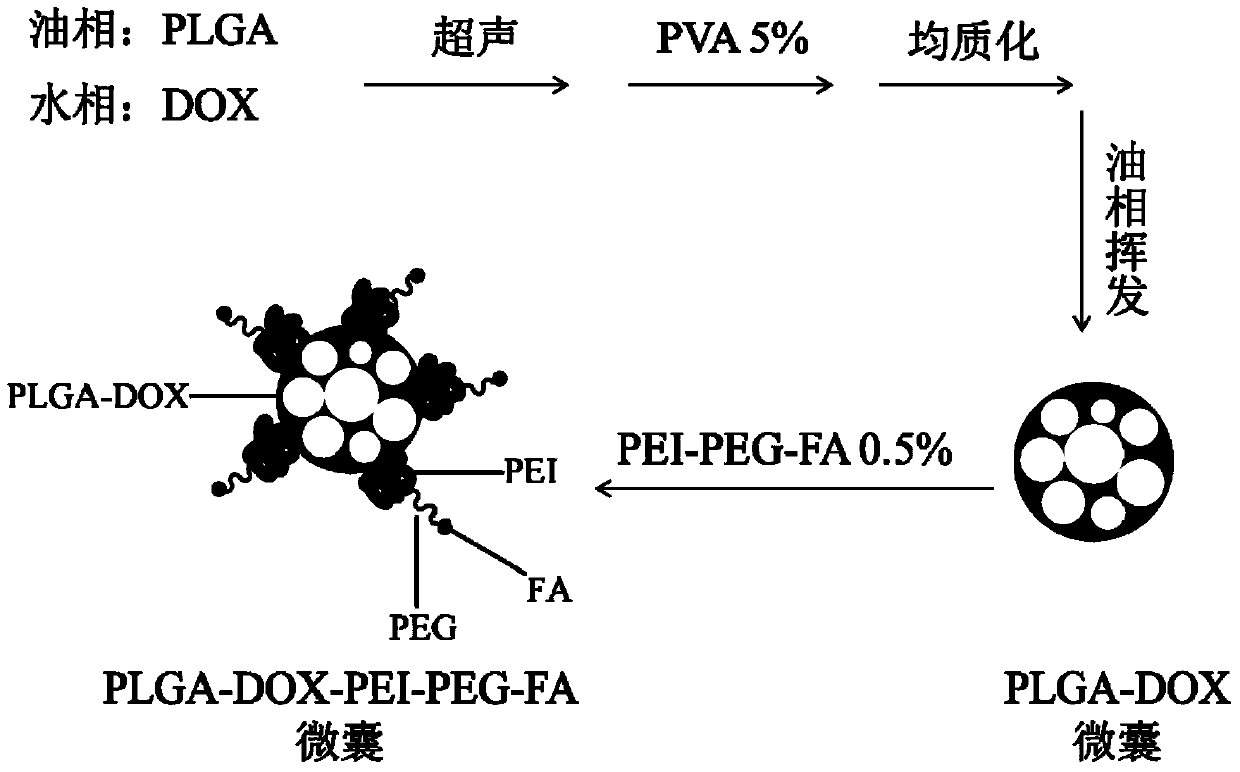
[0057] (1) with 2mL CH 2 Cl 2 Dissolve 100mg PLGA; dissolve 1mg DOX with 200μL ultrapure water, mix the organic phase and the aqueous phase, and ultrasonically treat with a cell disruptor for 30s in an ice-water bath to form a W / O emulsion;
[0058] (2) Transfer the W / O emulsion in step 1 into 10mL of 5% PVA aqueous solution, and shear it with a homogenizer (9500rpm, 5min) in an ice-water bath to make the droplets form a W / O / W emulsion with a relatively uniform size;
[0059] (3) Pour the W / O / W emulsion from step 2 into 80 mL of 2% isopropanol aqueous solution and magnetically stir for 1 h, then centrifuge (3500 rpm, 3 min), discard the supernatant, and wash the centrifuged residue with 90 mL of ultrapure water. Repeat the above centrifugation and washing operation for more than three times until the centrifugation liquid is clear and colorless, and redisperse the centrifugation residue in 1mL ultrapure water after centrifugation;
[0060] (4) Quickly add the centrifuge resi...
Embodiment 2
[0064] The in vitro DOX release of PLGA-DOX-PEI-PEG-FA hollow microcapsules was tested with pH7.4 PBS buffer and pH5.6 acetate buffer as drug release buffers. Take 10mg of PLGA-DOX-PEI-PEG-FA hollow microcapsules and disperse them with 3mL of buffer solution, then transfer them into a dialysis bag (Mw cut-off is 10000), put the dialysis bag into a glass bottle with a capacity of 25mL, and add 7mL of The same buffer makes the total volume of buffer inside and outside the dialysis bag 10mL. Put the above glass bottle into a constant temperature shaker at 37°C, take out 3mL of buffer at different time points (0.25, 0.5, 1, 2, 4, 8, 12, 24, 48, 72h) and add the same volume of the same type of buffer, For each material concentration, three sets of parallel samples were used to detect the standard deviation. Finally, the buffer solution taken out was tested for liquid absorbance at 481 nm under an ultraviolet-visible spectrophotometer, and the drug content in the buffer solution an...
Embodiment 3
[0066] The antitumor activity of the PLGA-DOX-PEI-PEG-FA hollow microcapsule complex was studied by detecting cell viability by MTT method, and PBS solution was used as a control. Human epithelial cancer cells (KB cells) were planted in a 96-well plate with a seeding density of 5×10 per well. 3KB cells. After culturing overnight, the PLGA-PEI-PEG-FA hollow microcapsules, PLGA-DOX-PEI-PEG-FA hollow microcapsules (microsphere concentrations of 3.375, 6.75, 12.5, 25, 50, 100 mg / L, DOX concentration of 0.025 , 0.05, 0.10, 0.20, 0.40, 0.80mg / L) and the same concentration of pure drug DOX were co-cultured with KB cells. After 48h, 20μL of MTT solution (5mg / mL) was added to each well and cultured for 4h, and then poured Take out the medium, add 200 μL DMSO, put it in a shaker and shake it slowly for 15 minutes, use a microplate reader to detect the absorbance at 490 nm, and make three sets of parallel samples for each material concentration to detect the standard deviation.
[0067...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com