Method for improving specific activity of microbial transglutaminase
A technology of transglutaminase specific enzyme activity and transglutaminase, applied in the direction of microorganism-based methods, transferases, botany equipment and methods, etc., can solve the problem that TGase cannot be secreted, affects TGase secretion and Catalytic activity and other issues, to achieve the effect of being suitable for industrial applications, improving enzyme activity, and improving specific enzyme activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1: Simulation of MTG crystal structure from Streptomyces hygroscopicus
[0040] Using the reported Streptomyces mobaraensis pro-TGase (PDB code: 3IU0) as a template (the similarity between the two amino acids is 73.1%), the online simulation software SWISS-MODEL was used to simulate the crystal structure of Streptomyces hygroscopicus TGase.
Embodiment 2
[0041] Example 2: Obtaining mutants
[0042] (1) Acquisition of mutant genes
[0043] The gene sequence of the short peptide (the underlined part in Table 1 is the base sequence of the short peptide) was designed on the primers, and the S.hygroscopicus pro-TGase expression plasmid pBB1-1011 was used as the template to perform full-plasmid PCR using site-directed mutagenesis technology Insert a short peptide into the C-terminus of the leader peptide. The plasmid pBB1-1011 was constructed based on the genome of Streptomyces hygroscopicus (obtained from the China Type Culture Collection, with an accession number of CCTCC NO: M203062) as a template. For the construction method, see the article Liu S, Zhang D, Wang M, Cui W , Chen K, Liu Y, Du G, Chen J, Zhou Z, (2011). The pro-region of Streptomyces hygroscopicus transglutaminase affects its secretion by Escherichia coli.FEMS Microbiol Lett324(2):98-105 in the expression plasmid pBB1- Construction method of 1010 / pBB1-1020. The prime...
Embodiment 3
[0052] Example 3: Detection of mutant enzyme properties
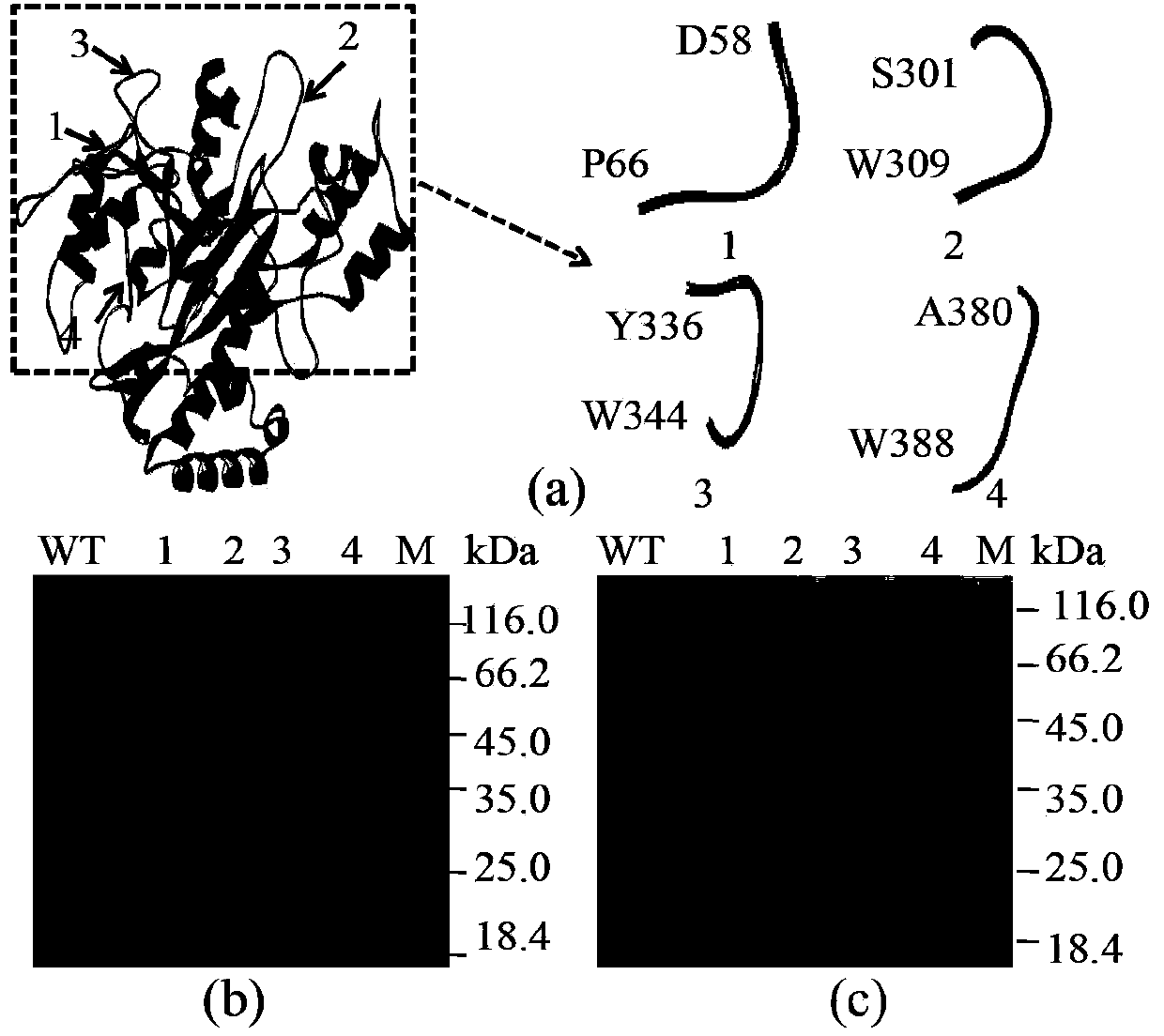
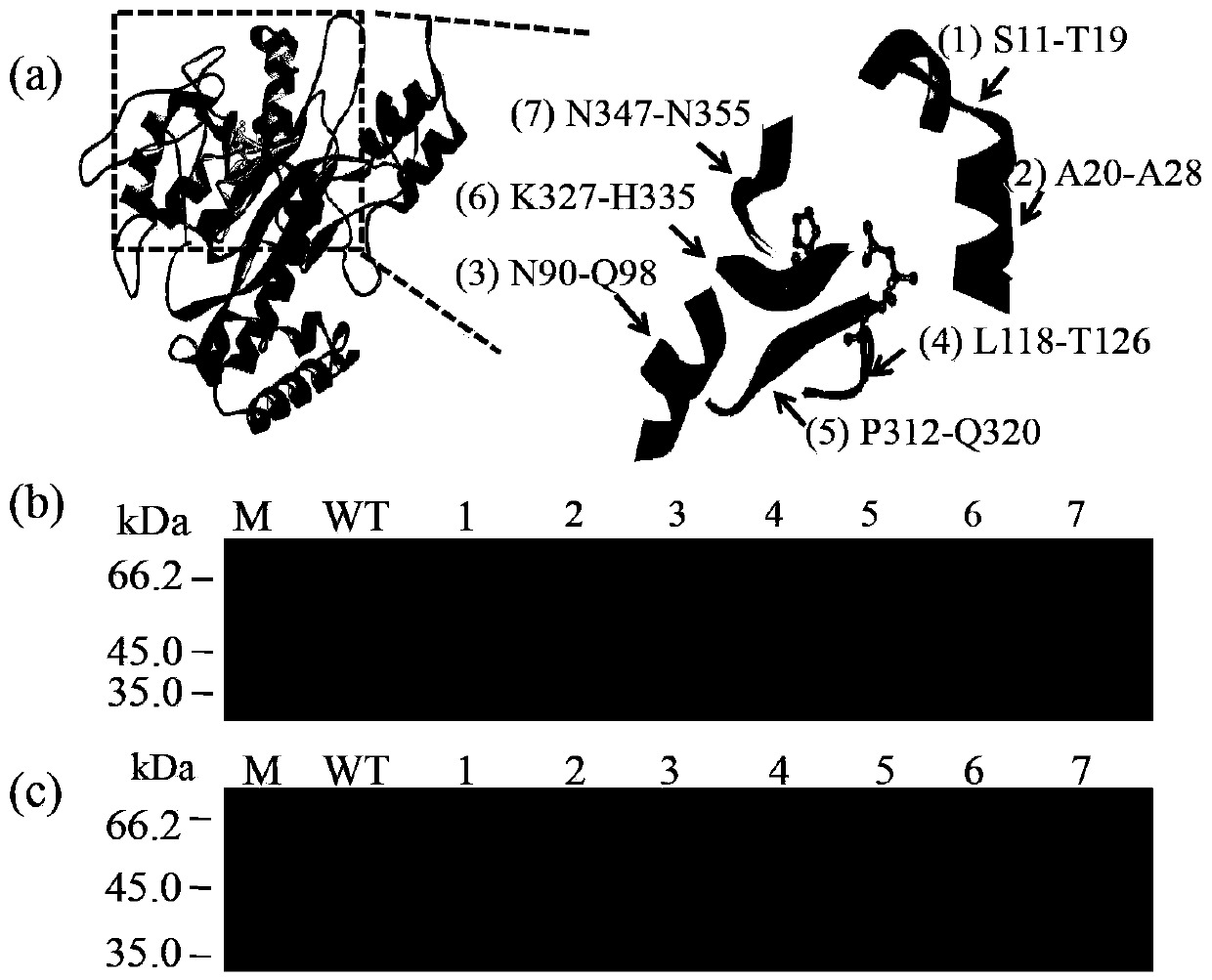
[0053] In order to make the leader peptide mutated and still be cleaved normally, in the present invention, the short peptide insertion site is selected before the leader peptide cleavage site (L53-F54), that is, before L53, and the short peptide is added to the N-terminus or C-terminus of the protein. The peptides are different. Through the formation of new interactions between the short peptide and its surrounding amino acids, TGase modification is realized, so short peptide selection is the key to modification. The present invention screens important short peptides from pro-TGase for TGase molecular modification, and the short peptides are limited to 9 amino acids.
[0054] (1) Choose 4 loops from the mature enzyme as insert short peptides
[0055] Since the C-terminus of the TGase leader peptide is a loop structure, firstly select 4 loops that are important for the catalytic activity of TGase from the mature enzyme as th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com