Process for reserve recycling and preparation of lithium nickel cobaltate from waste lithium battery as raw material
A technology of waste lithium batteries and lithium nickel cobalt oxide, which is applied in battery recycling, waste collector recycling, battery electrodes, etc., to achieve the effects of resource utilization, enhanced migration ability, and improved discharge performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
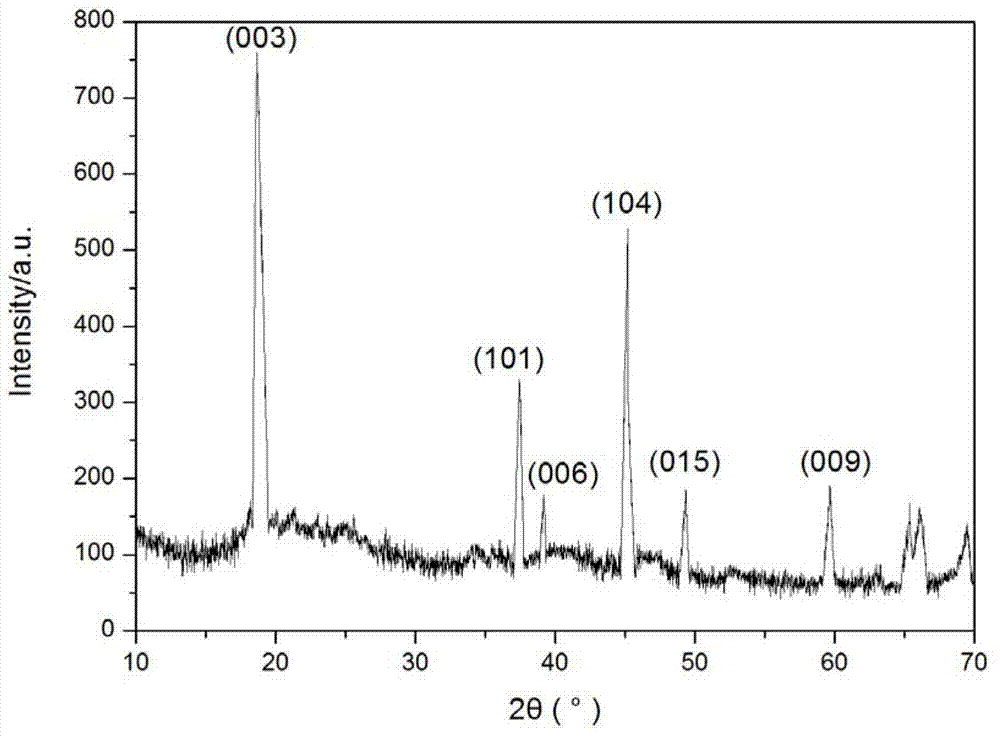
Image
Examples
Embodiment 1
[0035] A process for preparing lithium nickel cobalt oxide by reverse recovery of waste lithium batteries as raw materials, comprising the following steps:
[0036] (1) The battery cell was disassembled, and the positive electrode sheet was taken out; the positive electrode sheet was crushed, pyrolyzed in air at 400°C, and sieved with a 60-mesh standard sieve under vibration to obtain 1839 g of positive electrode powder.
[0037] (2) Dissolve the positive electrode powder in 10L2mol / L nitric acid, remove the insoluble matter by filtration, control the pH of the filtrate to 2, add 20L extractant, the components of the extractant are P204 with a volume fraction of 30% and P204 with a volume fraction of 70%. Sulfonated kerosene, the equilibrium time is 15min, then back extraction with 10L2mol / L nitric acid, the equilibrium time is 15min, the obtained solution is a mixed solution of nickel nitrate and cobalt nitrate, and the nickel ion concentration in the solution recorded by ICP ...
Embodiment 2
[0045] A process for preparing lithium nickel cobalt oxide by reverse recovery of waste lithium batteries as raw materials, comprising the following steps:
[0046] (1) The battery cell was disassembled, and the positive electrode sheet was taken out; the positive electrode sheet was crushed, pyrolyzed, and sieved with a 60-mesh standard sieve under vibration to obtain 1870 g of positive electrode powder.
[0047] (2) Dissolve the positive electrode powder in 10L2mol / L hydrochloric acid, remove the insoluble matter by filtration, control the pH of the filtrate to 2, add 20L extractant, the components of the extractant are P204 with a volume fraction of 30% and P204 with a volume fraction of 70%. Sulfonated kerosene, the equilibrium time is 15min, back extraction with 10L2mol / L hydrochloric acid, the equilibrium time is 15min, the obtained solution is a mixed solution of nickel chloride and cobalt chloride, and the nickel ion concentration in the solution is 0.70mol / L, the cob...
Embodiment 3
[0054] A process for preparing lithium nickel cobalt oxide by reverse recovery of waste lithium batteries as raw materials, comprising the following steps:
[0055] (1) The battery cell was disassembled, and the positive electrode sheet was taken out; the positive electrode sheet was crushed, pyrolyzed, and sieved with a 60-mesh standard sieve under vibration to obtain 1920 g of positive electrode powder.
[0056] (2) Dissolve the positive electrode powder in 10L2mol / L sulfuric acid, remove the insoluble matter by filtration, control the pH of the filtrate to 2, add 20L extractant, the components of the extractant are P204 with a volume fraction of 30% and P204 with a volume fraction of 70%. Sulfonated kerosene, the equilibrium time is 15min, then back extraction with 10L2mol / L sulfuric acid, the equilibrium time is 15min, the obtained solution is a mixed solution of nickel sulfate and cobalt sulfate, the nickel ion concentration in the solution is 0.82mol / L measured by ICP, T...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com