HIV (human immunodeficiency virus) antibody recognition reagent
A technology of human immunodeficiency and antibodies, which is applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve the problems of cumbersome operation and long reaction time, and achieve the effect of simple operation, simple operation and avoiding mistakes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1: Manufacture of HIV Antibody Confirmation Reagent
[0040] 1. Main materials
[0041] 1.1 HIV-specific antigens are gp160, gp120, p66, p55, p51, gp41, p31, p24, p17 of HIV-1 and gp36 of HIV-2: provided by Beijing Academy of Military Medical Sciences, HIV antigen expressed by genetic engineering; human IgG And mouse anti-human IgG: provided by Shenzhen Faipeng Biotechnology Co., Ltd.; NC membrane: product of Millipore Company of the United States; bovine serum albumin (BSA), polyethylene glycol (PEG) 20000, hydrolyzed protein: product of Sigma Company of the United States; others Reagents are chemical reagents of routine analytical grade.
[0042] 1.2 Testing instrument: Immunochromatographic knot reading recorder, model: NS3001, product of Tianjin Zhongxin Keju Biopharmaceutical Co., Ltd.
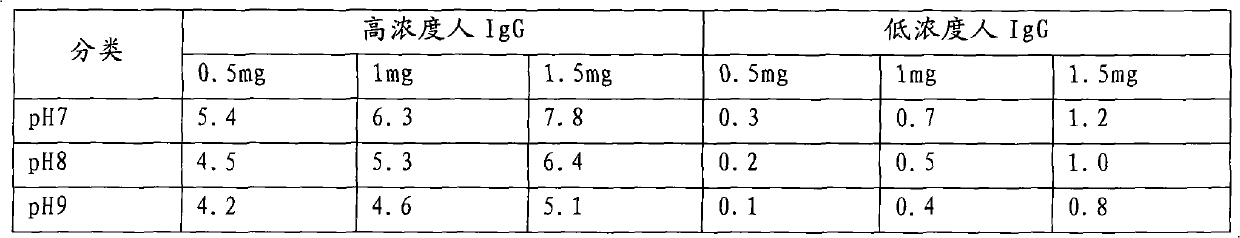
[0043] 1.3 Clinical serum: Obtained by the company in relevant hospitals, 19 HIV-1 positive samples and 1 HIV-2 positive sample have been confirmed by Singapore MP reagen...
Embodiment 2
[0063] Embodiment 2: clinical trial
[0064] 1. Main materials
[0065] 1.1 Examination of human immunodeficiency virus antibody confirmation reagents: the reagents were optimized and prepared according to Example 1.
[0066] 1.2 Confirmation reagent for control human immunodeficiency virus antibody: product of Singapore MP Company.
[0067] 1.3 Clinical samples: Obtained by the company in the relevant infection hospital, 100 HIV-positive samples, including 95 HIV-1 positive samples, positive for HIV antibody in ELISA preliminary screening; 5 HIV-2 positive samples, confirmed positive samples; 500 negative samples The samples were collected from healthy people, and the comprehensive clinical test was judged to be negative.
[0068] 2. Detection method
[0069] Carry out according to the method of the respective product instructions, determine the test results, and conduct analysis.
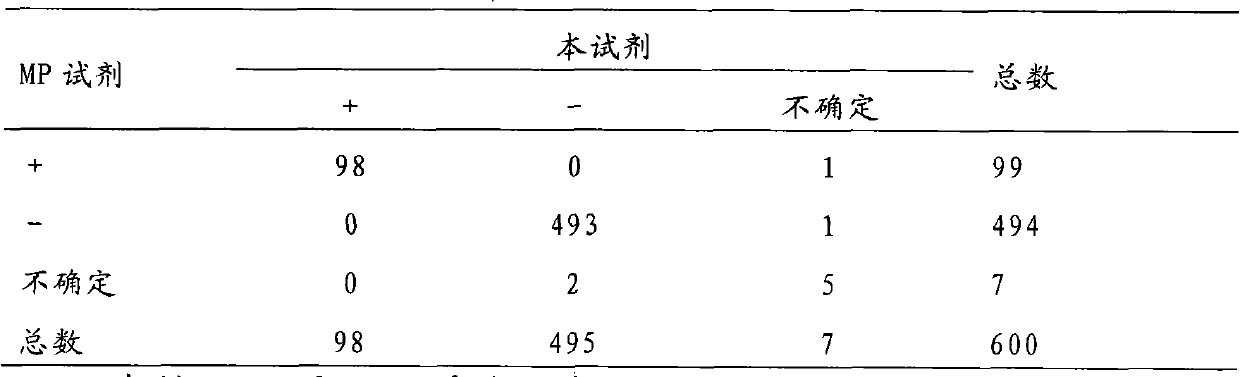
[0070] 3. Results: The test results are as follows:
[0071] Table 3: Comparison of test ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com