Human umbilical cord mesenchymal stem cell culture medium
A technology of stem cells and culture medium, applied in the field of human umbilical cord mesenchymal stem cell culture medium, can solve the problems of stem cell activity, short form retention time, low viability rate, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] In this example, the basal medium is mixed with the following three concentrations of cysteine-rich secreted acidic protein, and the three specific concentrations are shown in Table 1a:
[0022] Table 1a
[0023] Numbering
concentration
①
1mg / L
②
1×10 -3 mg / L
③
1×10 -6 mg / L
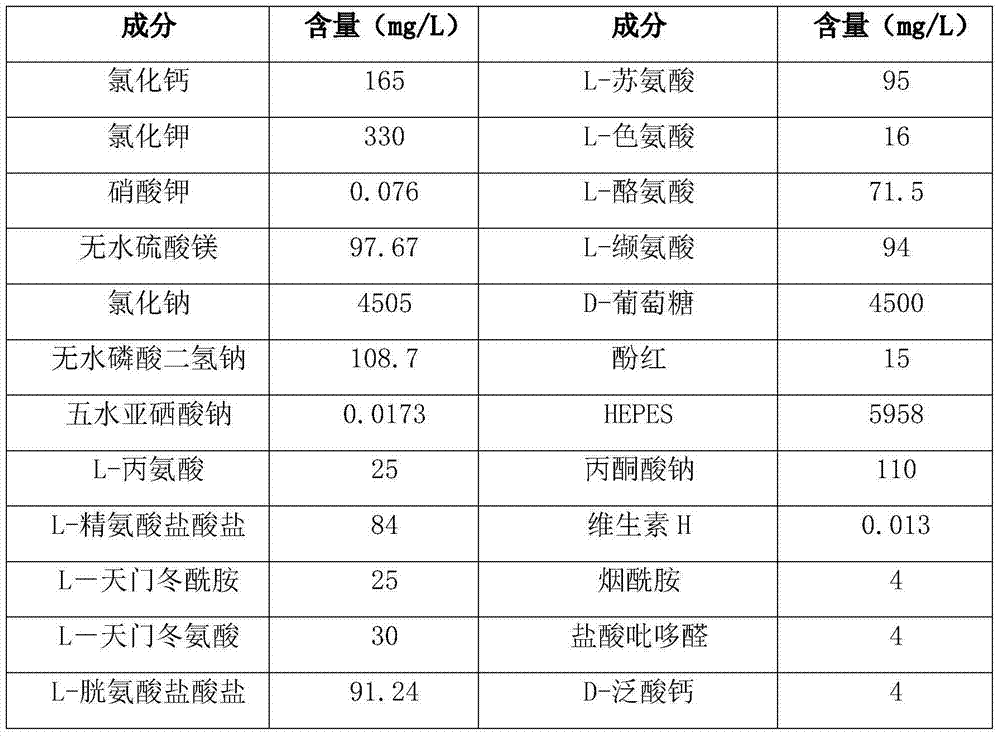
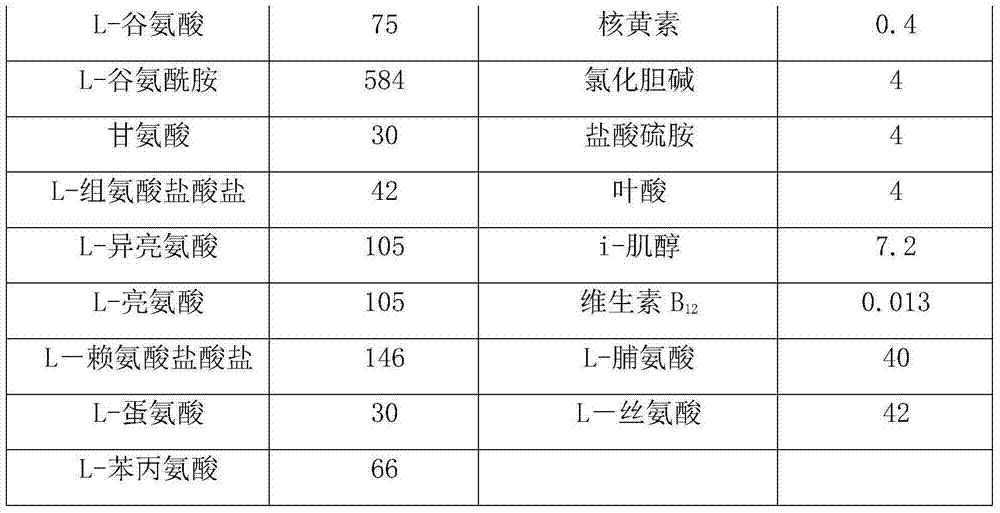
[0024] Wherein, the composition of basal medium is shown in Table 1b:
[0025] Table 1b
[0026]
[0027]
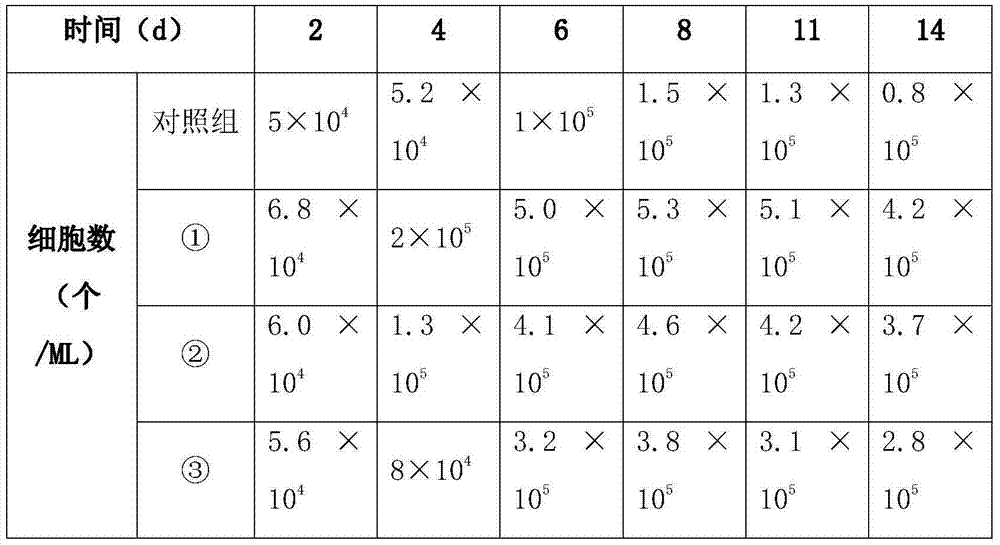
[0028] Inject 5×10 in each ML culture medium 4 The cells were cultured, wherein three different concentrations of medium in Table 1a were used as the experimental group, and the basal medium was used as the control group. The experimental group and the control group were tested, and the test results were shown in Table 1c:
[0029] Table 1c
[0030]
[0031] When the number of cells in the 1ML culture medium was the largest, the cells were detected, and it was found that: the cells in the experimental group were all long s...
Embodiment 2
[0034] The difference between this example and Example 1 is that the composition of the protein added to the basal medium is different, and its specific composition is shown in Table 2a:
[0035] Table 2a
[0036]
[0037] Inject 5×10 in each ML culture medium 4 The cells were cultured, wherein three different concentrations of medium in Table 1a were used as the experimental group, and the basal medium was used as the control group. The experimental group and the control group were tested, and the test results were shown in Table 2b:
[0038] Table 2b
[0039]
[0040] When the number of cells in the 1ML culture medium was the largest, the cells were detected, and it was found that the cells in the experimental group were all long spindle-shaped, the nuclei were round or oval, and the average activity rate reached 97.3%; while in the control group The cells were spindle-shaped and fan-shaped, and the shape of the cells changed, and the nucleus in the fan-shaped cells ...
Embodiment 3
[0043] The difference between this example and Example 1 lies in the composition and concentration of the basal medium and protein. The specific composition of the basal medium is shown in Table 3a, and the specific composition of the protein is shown in Table 3b.
[0044] Table 3a
[0045] Element
Content (mg / L)
Element
Content (mg / L)
100
L-serine
30
400
20
48.84
5
6000
L-tyrosine
20
300
L-valine
20
L-Arginine
200
D-glucose
2000
50
Glutathione (reduced form)
1
20
3000
65.15
Phenol red
5
20
Vitamin H
0.2
300
Calcium D-pantothenate
...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com