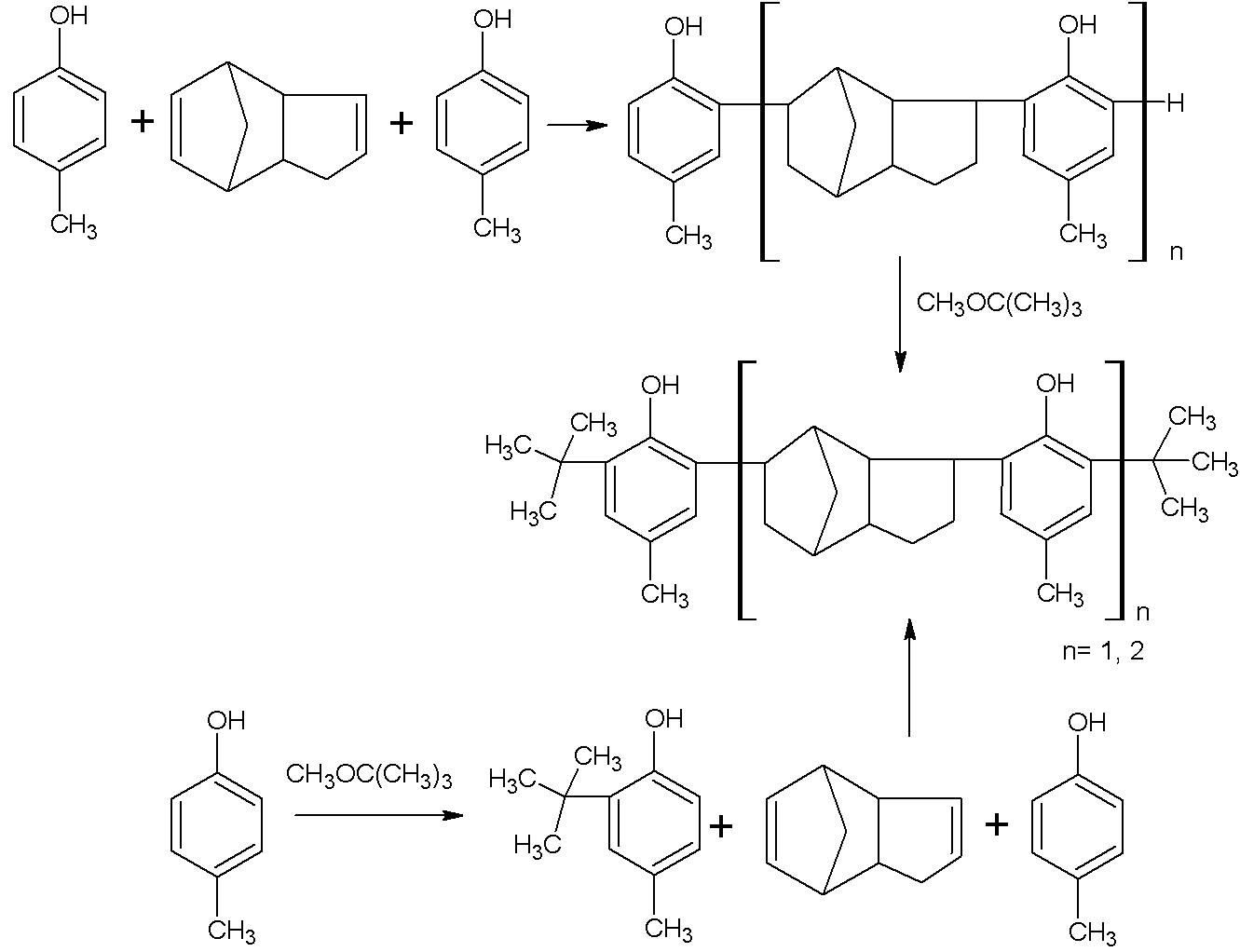
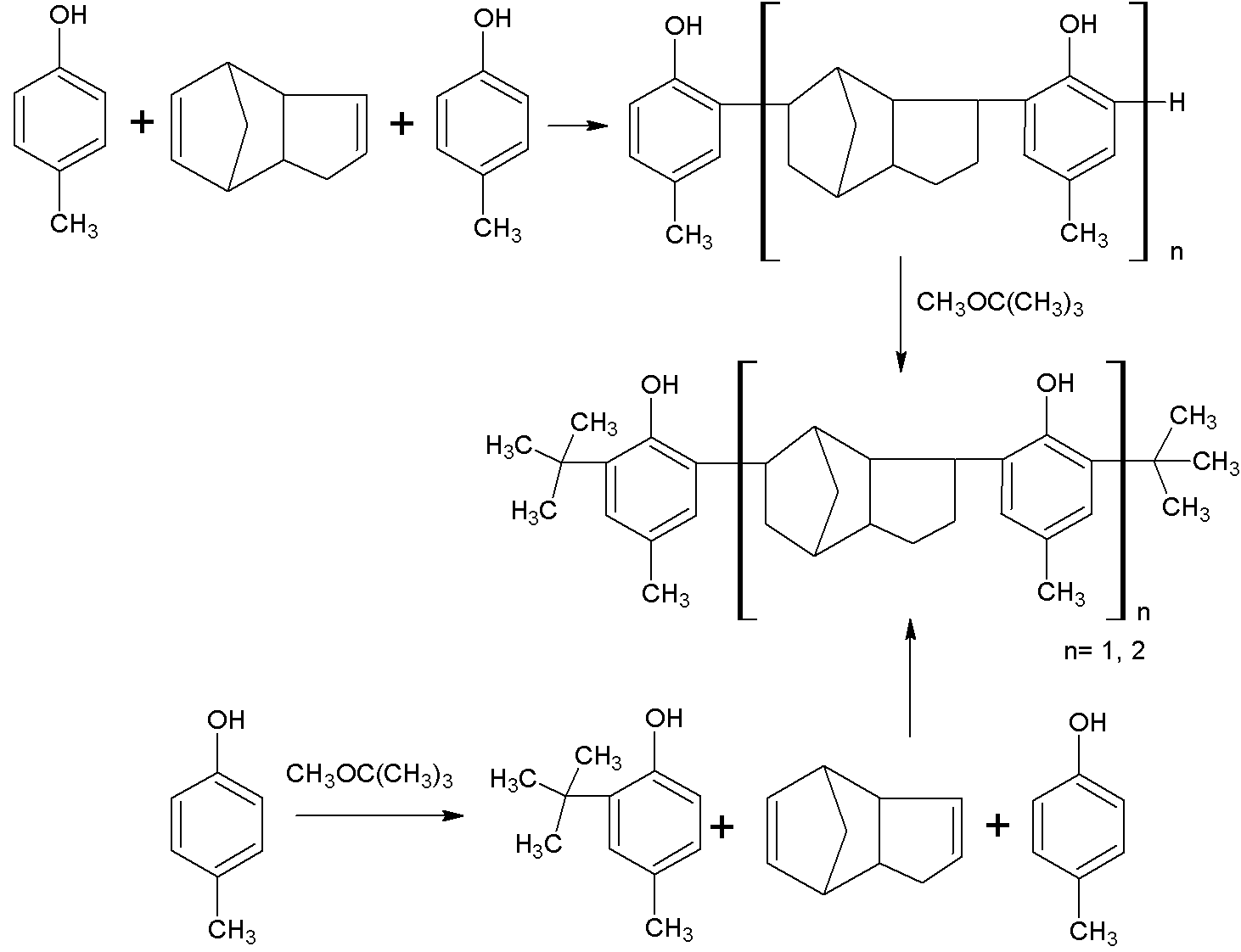
Method for producing aggregate-type hindered phenol antioxidant
A hindered phenol antioxidant, polymerization type technology, applied in the field of chemical synthesis, can solve problems such as cumbersome process, achieve the effects of clean and environmentally friendly process, low ash content, and simple treatment process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Under the protection of nitrogen, add 132Kg xylene and 162Kg (1.5Kmol) p-cresol into the 1500L enamel reaction kettle, stir and raise the temperature to 140°C to remove the water in the solution. Then lower the temperature to 80° C. and add 1.32 kg of organic composite sulfonic acid catalyst dropwise. After stirring evenly, add 132Kg (1 Kmol) of dicyclopentadiene dropwise for 3 hours. After the dropwise addition was completed, the temperature was continued to rise to 150°C for 9 hours. The temperature of the reaction feed liquid was lowered to 50°C, and 132Kg (1.5Kmol) tert-butyl methyl ether was added dropwise for 2 hours. After the dropwise addition, the temperature was raised to 150° C. to continue the reaction for 9 h.
[0028] After the reaction was completed, the temperature was lowered to room temperature. Then add 100Kg saturated brine to the reaction kettle to wash twice, stir, and let stand to separate layers. Then wash the organic layer with 100Kg 5% sodi...
Embodiment 2
[0030] Under the protection of nitrogen, add 660Kg toluene and 162Kg (1.5Kmol) p-cresol into the 2000L enamel reaction kettle, stir and raise the temperature to 140°C to remove the water in the solution. Then lower the temperature to 80° C. and add 132 Kg of organic composite sulfonic acid catalyst dropwise. After stirring evenly, add 132Kg (1Kmol) of dicyclopentadiene dropwise for 3 hours. After the dropwise addition was completed, the temperature was continued to rise to 150°C for 9 hours of reaction. The temperature of the reaction feed liquid was lowered to 50°C, and 132Kg (1.5Kmol) tert-butyl methyl ether was added dropwise for 2 hours. After the dropwise addition was completed, the temperature was raised to 150° C. to continue the reaction for 9 h.
[0031] After the reaction was completed, the temperature was lowered to room temperature. Then add 100Kg saturated brine to the reaction kettle to wash twice, stir, and let stand to separate layers. Then wash the organic...
Embodiment 3
[0033] Under the protection of nitrogen, add 660Kg chlorobenzene and 216Kg (2Kmol) p-cresol into the 1500L enamel reaction kettle, stir and raise the temperature to 140°C to remove the water in the solution. Then lower the temperature to 80° C. and add 1.32 kg of organic composite sulfonic acid catalyst dropwise. After stirring evenly, add 132Kg (1Kmol) of dicyclopentadiene dropwise for 3 hours. After the dropwise addition was completed, the temperature was continued to rise to 150°C for 9 hours of reaction. The temperature of the reaction feed liquid was lowered to 50°C, and 176Kg (2Kmol) of tert-butyl methyl ether was added dropwise for 2 hours. After the dropwise addition was completed, the temperature was continued to rise to 150° C. for 9 h.
[0034] After the reaction was completed, the temperature was lowered to room temperature. Then add 100Kg saturated brine to the reaction kettle to wash twice, stir, and let stand to separate layers. Then wash the organic layer w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com