new anti-dr5 antibody
A technology of antibodies and chimeric antibodies, applied in the direction of antibodies, antibody medical components, antibody mimics/stents, etc., can solve problems such as no correlation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0394] [Example 1] Production of mouse antibody B273
[0395] 1)-1 Production of human DR5 protein (human DR5 extracellular domain / human Fc fusion protein)
[0396] 1)-1-1 Production of human DR5 extracellular domain expression vector
[0397] A vector expressing human DR5 protein (isoform 2: NP_671716) was constructed by inserting a gene in which the extracellular domain of human DR5 was fused to the human IgG1 / Fc region downstream of the CMV promoter.
[0398] 1)-1-2 Production of human DR5 protein
[0399] Introduction of the expression vector into 293 FreeStyle cells and collection of the culture supernatant were performed by Invitrogen Corporation (now Life Technologies Japan Ltd.).
[0400] 1)-1-3 Purification of human DR5 protein
[0401] The culture supernatant obtained in b) above was purified using protein A affinity column chromatography. 5 L of the culture supernatant was applied to "HiTrap Protein AFF" (GE Healthcare Bio-Sciences Co., Ltd., catalog number 17-5...
Embodiment 2
[0428] [Example 2] Cloning of mouse antibody B273 gene and production of human chimeric antibody gene
[0429] 2)-1 Cloning and sequence determination of mouse antibody B273 cDNA
[0430] 2)-1-1 Determination of N-terminal amino acid sequences of heavy chain and light chain of mouse antibody B273
[0431]In order to determine the N-terminal amino acid sequences of the heavy and light chains of the mouse antibody B273, the mouse antibody B273 purified in Examples 1-8 was separated by SDS-PAGE. After separation, the protein in the gel was transferred from the gel to a PVDF membrane (pore size: 0.45 μm, manufactured by Invitrogen Corporation). The membrane was washed with wash buffer (25 mM NaCl, 10 mM sodium borate buffer, pH 8.0) and thereafter stained by immersion in dye solution (50% methanol, 20% acetic acid, 0.05% Coomassie brilliant blue) for 5 minutes, followed by 90% Methanol decolorizes. The band corresponding to the heavy chain (band with smaller mobility) and the b...
Embodiment 3
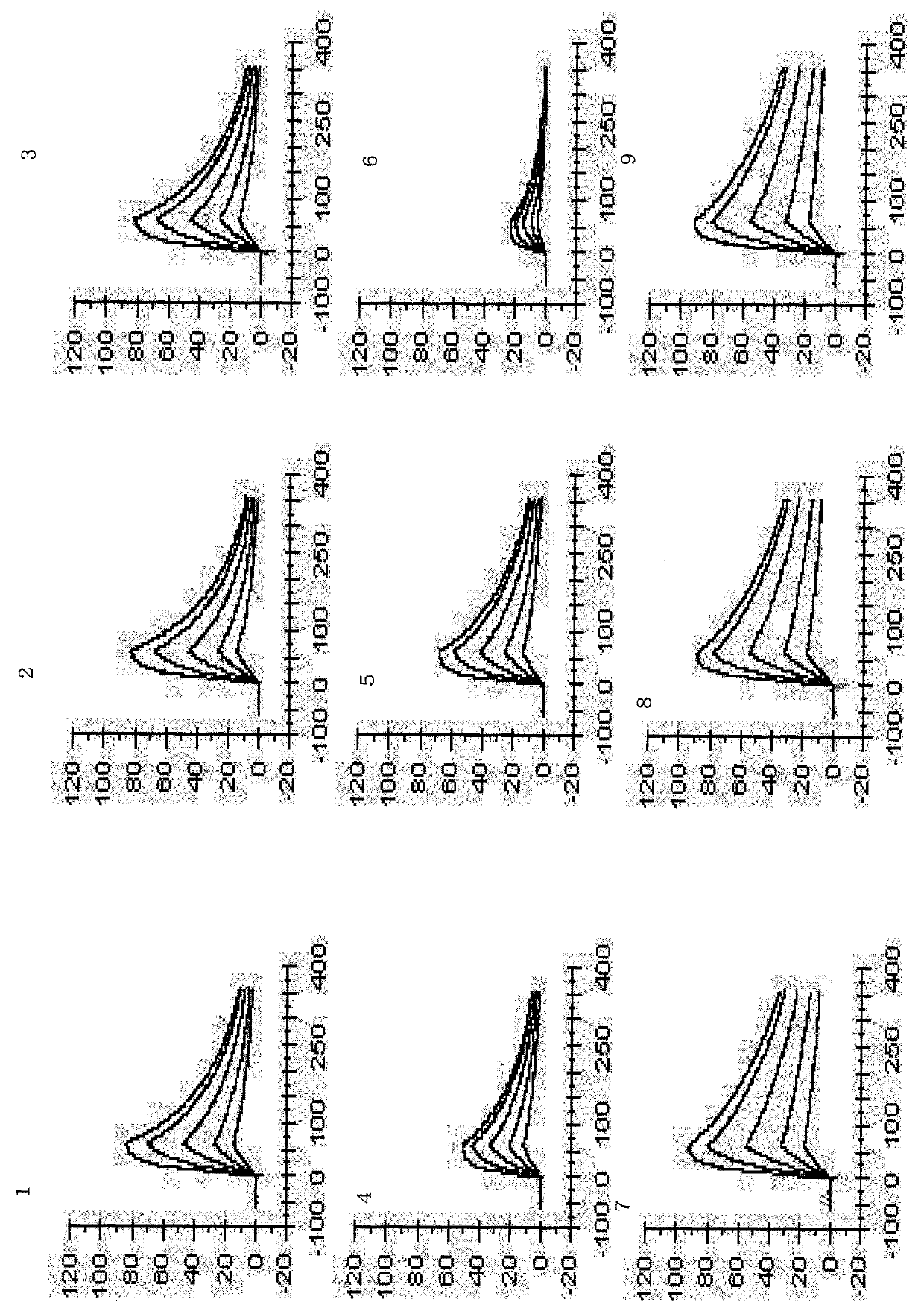
[0472] [Example 3] Measurement of human chimeric B273 (cB273) antibody activity (in vitro)
[0473] 3) Study on the selective binding properties of -1 cB273 antibody to the extracellular domain of human DR5
[0474] The binding properties of cB273 to human TRAIL R1-R4 and mouse TRAIL R2 extracellular domain proteins (manufactured by R&D Systems, Inc.) were investigated by the direct ELISA method described below. First, each of the ectodomain proteins of TRAIL R was diluted to 1 μg / ml with PBS, and the diluted solution was distributed in an immunoplate (manufactured by Nunc, Inc., #442404) at 50 μl / well, and the plate was incubated at 4 The protein was thus adsorbed to the plate by standing overnight at ℃. The next day, the liquid in each well was removed, and each well was washed once with PBS. Thereafter, in order to suppress non-specific adsorption of proteins, 200 μl / well of PBS containing 3% fetal bovine serum was dispensed, and the plate was allowed to stand at room tem...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com