Method for enriching and separating helicobacter pylori
A Helicobacter pylori enrichment technology, applied in the biological field, can solve the problems of separation failure, poor monodispersity of micron magnetic beads, and large concentration of miscellaneous bacteria, so as to increase the chance of contact, shorten the separation time, and improve the capture efficiency.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
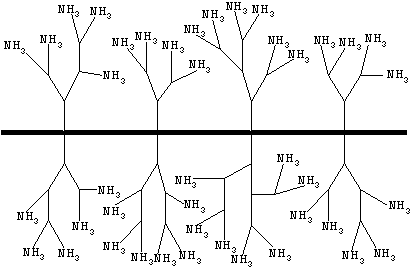
[0028] 1. The dendritic hyperbranched polymer-antibody complex is prepared according to the following steps:
[0029] (1) Weigh 4.5 mg of dendritic polyamide-amine amined with dendritic hyperbranched polymer, and dissolve it in 4 mL of phosphate buffer (PBS, 0.01 mol / L, pH 8.0), stir and add 25% pentane. 545 μL of dialdehyde aqueous solution to make the final concentration of glutaraldehyde 3%. React at room temperature for 3.5 h at a rotating speed of 150 r / min on a shaker;
[0030] (2) Add Helicobacter pylori dropwise to the above solution HP The specific antibody is 12 mg, and its final concentration is about 3 mg / mL. React at room temperature for 24 hours at a speed of 150 r / min on a shaker;
[0031] (3) The above solution was spin-dried solvent under reduced pressure, dissolved in deionized water, and dialyzed in PBS and deionized water for 1 d; after the dialysis, the obtained solution was freeze-dried.
[0032] 2. The long-chain biotin-dendritic hyperbranched polymer...
Embodiment 2
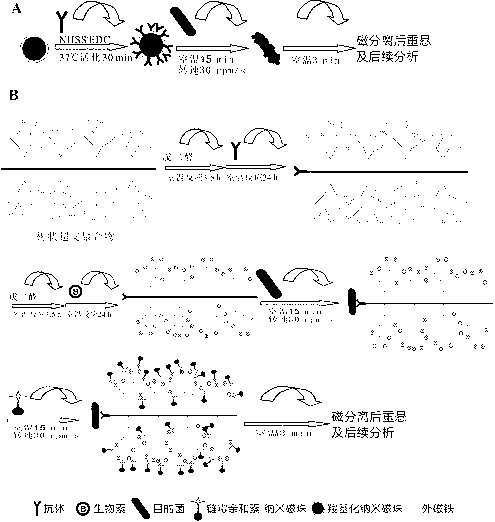
[0038] Example 2 Enrichment effect experiment
[0039] (1) Take 1 mL concentration as 10 4 The cfu / mL Helicobacter pylori was centrifuged at 12000 rpm for 5 min in a 1.5 mL sterile centrifuge tube, the supernatant was discarded, and resuspended in an equal volume of sterile PBS solution.
[0040] (2) Enrichment and capture: The technical solution group of the present invention (the dendritic hyperbranched polymer group co-modified by Helicobacter pylori antibody and long-chain biotin), the Helicobacter pylori specific antibody-modified nanomagnetic bead group, and Helicobacter pylori Micron magnetic beads modified by bacteria-specific antibodies enrich the target bacteria.
[0041] (3) After magnetic separation, pour the supernatant into a sterile centrifuge tube, and the separated immunomagnetic beads that trap Helicobacter pylori are washed twice with PBST, mixed well, and resuspended with 1 mL sterile PBS solution Immunomagnetic bead complex.
[0042] (4) Calculation of capture r...
Embodiment 3
[0055] Example 3 Enrichment and capture experiment
[0056] The conventional magnetic stand separation time is 30min, and the rest is the same as in Example 2.
[0057] The capture rate of each group is as follows:
[0058] The capture rate of Helicobacter pylori specific antibody modified micron magnetic beads The capture rate of Helicobacter pylori specific antibody modified nano magnetic beads Capture rate of dendritic hyperbranched polymer co-modified with Helicobacter pylori antibody and long-chain biotin 53.1% 34.8% 91.0%
[0059] The experimental results show that the separation of 3 minutes in Comparative Example 2 and when the separation time reaches 30 minutes, the capture efficiency of the three groups is improved, especially the Helicobacter pylori-specific antibody-modified nanomagnetic bead group has the most obvious improvement. This indicates that the capture efficiency of the nanomagnetic bead group can be greatly improved by prolonging the time, but it is s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com