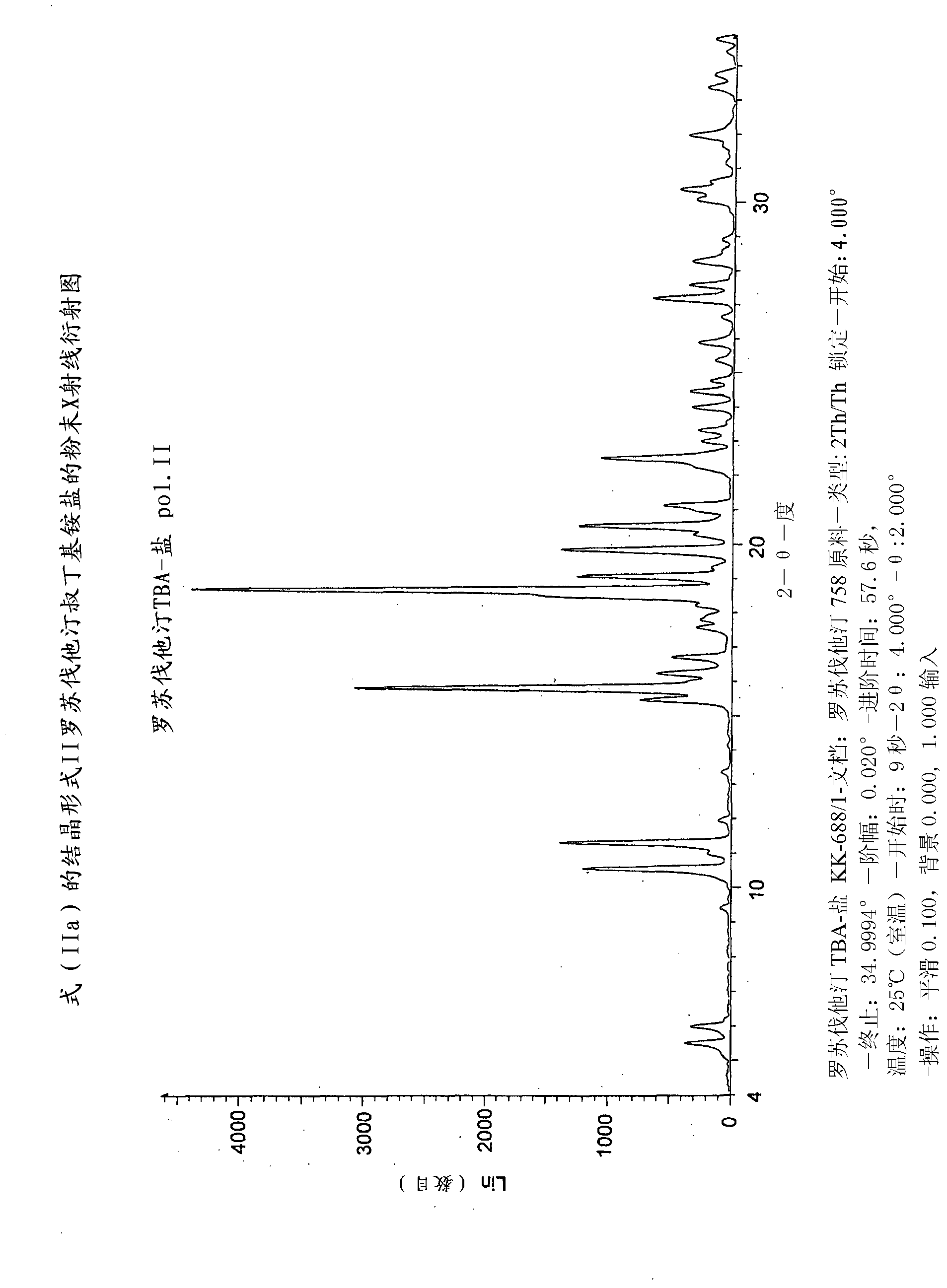
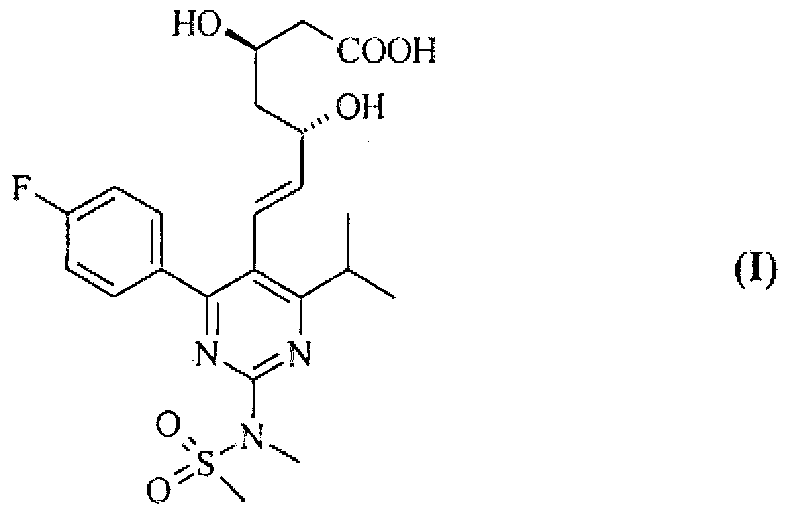
Method for preparing rosuvastatin salts
一种罗苏伐他汀、汀铵盐的技术,应用在制备所述中间体领域,能够解决没有公开等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082] Preparation of rosuvastatin tert-butylammonium salt starting from rosuvastatin n-butylamide
[0083] Method "A" : to 800cm 3 Add 16.1g (0.03mol) of rosuvastatin n-butylamide in the autoclave, 644cm 3 of water and 43.9g (63.3cm 3 ; 0.60 mol) of tert-butylamine. The reaction mixture was stirred at 120°C for 24 hours. The mixture was cooled to room temperature, diluted with 2-propanol, and evaporated in vacuo. The residue was stirred in a mixture of tert-butylmethyl ether and heptane (2:5, v / v), and the crystals were filtered. In this way, 16.2 g (99%) of rosuvastatin TBA salt were obtained. The crude salt was boiled in acetonitrile / 2-propanol (8.6:1, v / v) mixture followed by further stirring at room temperature, filtered, washed and dried. The product thus obtained was boiled in acetonitrile, 2-propanol was added to the boiled mixture, decolorized with carbon and filtered. Precipitated crystals were filtered, and washed with acetonitrile. 10.9 g (66%) of rosuvast...
Embodiment 2
[0103] Preparation of rosuvastatin tert-butylammonium salt starting from rosuvastatin N,N-dimethylamide
[0104] Method "A": to have 50cm 3 0.89g (1.75mmol) of rosuvastatin N, N-dimethylamide, 35.6cm 3 of water and 2.56g (3.7cm 3 ; 3.5 mmol) of tert-butylamine. The reaction mixture was stirred at 120°C for 16 hours. The mixture was cooled to room temperature, the reaction mixture was diluted in portions with ethanol, and evaporated in vacuo. The evaporation residue was dissolved in tert-butyl methyl ether and heptane (2:5 v / v, 4 cm 3 ), and the crystals were filtered. In this way, 0.87 g (90%) of rosuvastatin TBA were obtained. The crude salt was recrystallized from acetonitrile / 2-propanol. This gave 0.58 g (60%) of rosuvastatin TBA salt with a purity (HPLC) greater than 99.5%.
[0105] Method "B": To have 50cm 3 0.89g (1.75mmol) of rosuvastatin N, N-dimethylamide, 35.6cm 3 Water-ethanol 9:1 (v / v) solvent mixture and 2.56g (3.7cm 3 ; 3.5 mmol) of tert-butylamine. T...
Embodiment 3
[0107] Preparation of rosuvastatin tert-butylammonium salt starting from rosuvastatin pyrrolidinylamide
[0108] To have 50cm 3 Fill the rosuvastatin pyrrolidinyl amide of 0.88g (1.65mmol) in the autoclave of volume, 35.2cm 3 of water and 2.41g (3.5cm 3 ; 3.3 mmol) of tert-butylamine. The mixture was stirred at 120° C. for 16 hours, cooled to room temperature, diluted portionwise with ethanol, and evaporated in vacuo. The residue was stirred in a mixture of ether-hexane (1:1, v / v), and the crystals were filtered. The product thus obtained was recrystallized from acetonitrile / 2-propanol (2:1, v / v). Rosuvastatin TBA was obtained in a yield of 0.55 g (60%) with a purity (as assessed by HPLC) of greater than 99.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com