Hederagenin amide derivative and preparation method and application thereof
A technology of helexin and aglycone amide, applied in the field of medicine, can solve the problems of low antidepressant biological activity, poor water solubility and the like, and achieve the effects of being beneficial to recovery, good antidepressant and good effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0035] The present invention will be further described in detail below in conjunction with the accompanying drawings and embodiments.
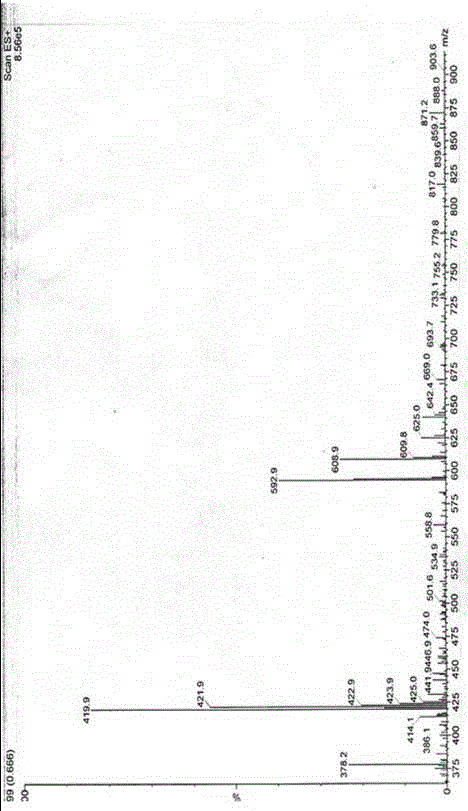
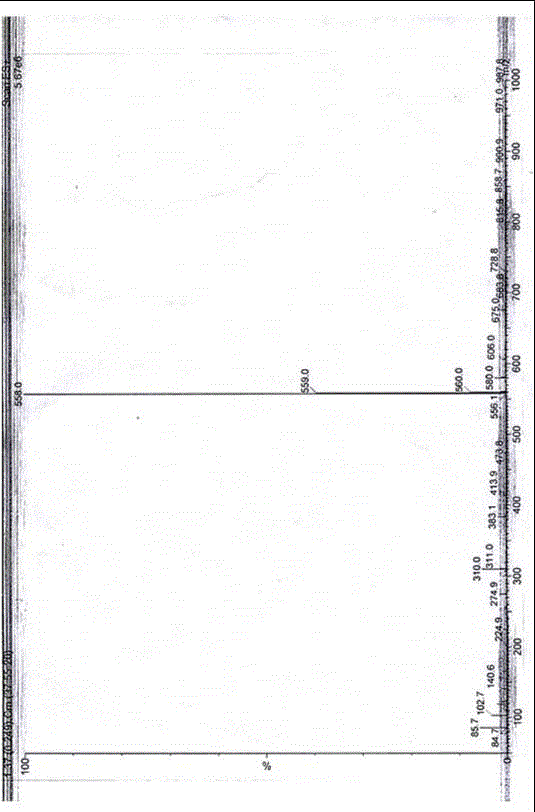
[0036] A helexin amide derivative, wherein: the hedera amide derivative is N-(3-dimethylaminopropyl)-helexin-17-carboxamide, abbreviated as HGA, and the general formula is .
[0037] The 3-dimethylaminopropyl group in N-(3-dimethylaminopropyl)-helexin-17-carboxamide is formed by introducing 3-dimethylaminopropylamine at the 28-position of helexin.
[0038] When preparing N-(3-dimethylaminopropyl)-helexin-17-carboxamide of the present invention, the experimental instruments and reagents that need to be used are:
[0039] Determination by nuclear magnetic resonance (TMS is internal standard, CD 3 OD is the solvent); rotary evaporator, electronic balance, circulating water vacuum pump, liquid chromatograph, quaternary low-pressure gradient pump, automatic sampler, diode array detector, chromatographic workstation, 4.6mm×250mm Agela 18 C chrom...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com