Preparation method of azoxystrobin
一种嘧菌酯、乙酸丁酯的技术,应用在嘧菌酯的制备领域,能够解决环境污染、分离回收产物的过程困难、增加生产成本等问题,达到简化结晶过程、操作简便、避免污染的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
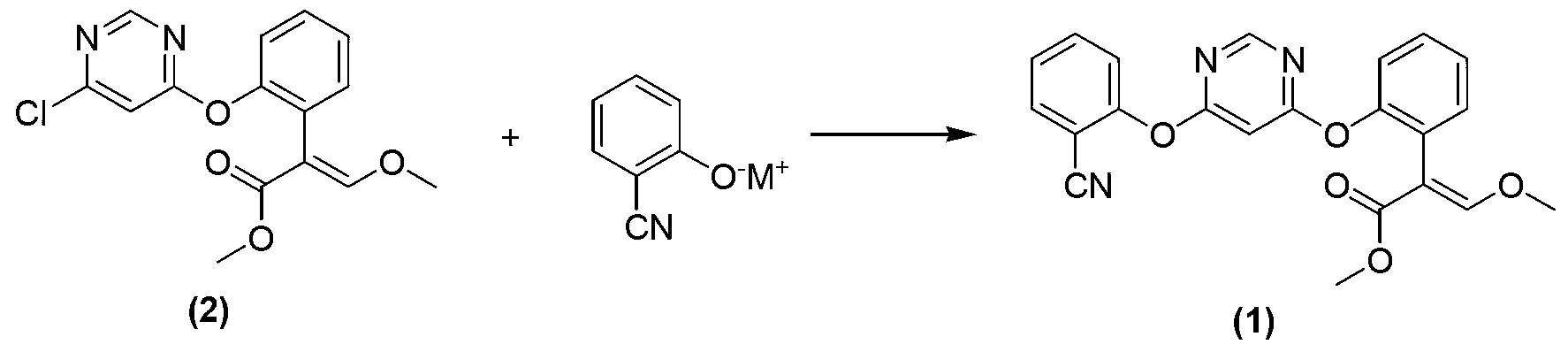
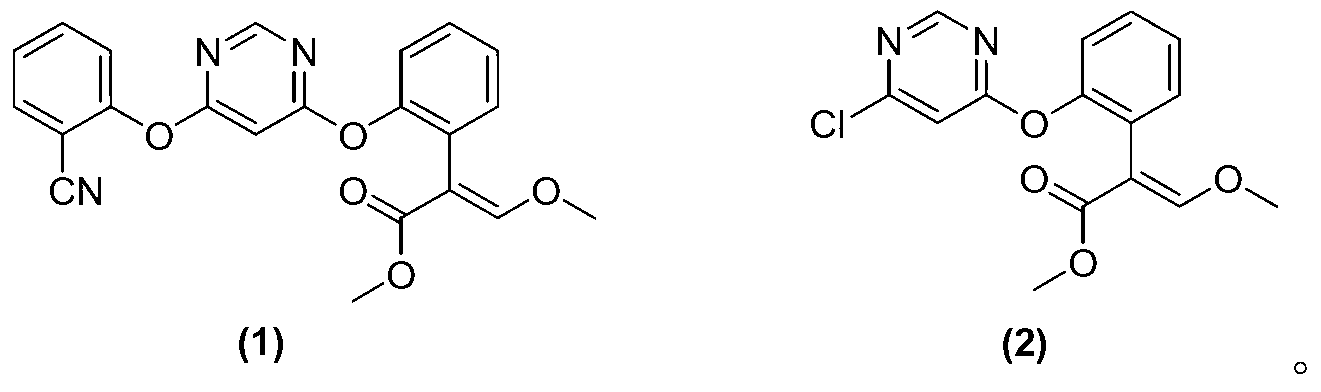
[0013] The present invention provides a method for preparing azoxystrobin with the structure shown in formula (1), the method comprising: a) combining the compound with the structure shown in formula (2) with 2-cyanophenol and / or its salt as Under the catalysis of the azabicyclic tertiary amine compound and / or its salt of the catalyst, an etherification reaction occurs in a butyl acetate medium to obtain a butyl acetate solution containing azoxystrobin; b) the azoxystrobin-containing The butyl acetate solution of the ester is cooled, and the azoxystrobin of the structure shown in formula (1) is separated out from the butyl acetate,
[0014]
[0015] In the production method of azoxystrobin according to the present invention, the azabicyclic tertiary amine compound is at least one of the compounds represented by formula (3), the compound represented by formula (4) and the compound represented by formula (5). A sort of;
[0016]
[0017] Among them, in formula (3), R 1 a...
Embodiment 1
[0045] This example is used to illustrate the preparation method of azoxystrobin provided by the present invention.
[0046] Add 0.105mol of 2-cyanophenol, 0.11mol of anhydrous potassium carbonate, and 100ml of butyl acetate into a 500ml glass-lined reactor, heat up to 70°C under stirring, and add 0.1mol of (E)-2-[2-(6- Chloropyrimidine-4-methoxy)phenyl]-3-methoxymethyl acrylate (the compound shown in the aforementioned formula (2), purchased from Bailingwei Company, with a purity of 95%) and a catalyst, the catalyst is 0.004mol2- Methyl-1,4-diazabicyclo[2.2.2]octane (purchased from Qingdao Hanbing Chemical Co., Ltd., with a purity of 99%), the reaction mixture was heated to 105°C and kept for 4 hours for reaction, and The situation that gas chromatographic detection reaction is carried out, when gas chromatographic display (E)-2-[2-(6-chloropyrimidine-4-methoxy) phenyl] the area normalization of -3-methoxymethyl acrylate is less than Add 50ml of water to the system at 1%, st...
Embodiment 2
[0050] Azoxystrobin was prepared according to the method of Example 1, except that the amount of butyl acetate added was 60 ml, and the reaction temperature was 80°C. 36.7 g of a yellowish-white solid were obtained, melting point: 115-116°C.
[0051] Detect 10mg solid product with NMR and MS, the data is 1 H NMR (500NMR, CDCl 3 ):δ3.64(s,3H),3.75(s,3H),3.62(s,2H),6.42(d,1H),7.22(q,1H),7.29-7.43(m,5H),7.49( s, 1H), 7.66(m, 1H), 7.10(q, 1H), 8.40(d, 1H), the data is in full agreement with the theoretical value of the compound shown in formula (1), proving that the product is such as formula ( 1) Compounds shown.
[0052] The calculated yield of the product is 94.4%, and the purity is 99.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com