Fluoroalcohol containing molecular photoresist materials and processes of use
A photoresist and molecular glass technology, applied in the field of molecular glass photoresist, can solve the problems of high dissolution rate and incompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0085] Example 1: In this example, 4-(1,1,1,3,3,3-hexafluoro- 2-Hydroxy-2-propyl)phenylresorcinolcalix[4]arene (structure 1 shown below).
[0086] Structure 1
[0087]
[0088] 4-(1,1,1,3,3,3-hexafluoro-2-hydroxyl-2-propyl)benzaldehyde (5 g, 0.018 mol), resorcinol (2 g, 0.018 mol) and Methanol (20 mL) was placed in a round bottom flask equipped with a stirrer. At room temperature, 5 ml of concentrated hydrochloric acid was added to the mixture, after which the reaction mixture was refluxed at 70°C for 18 hours. Completion of the reaction is determined by the disappearance of the carbonyl absorption peak of the aldehyde starting material using NMR or IR spectroscopy. The reaction solvent, methanol, was evaporated using a rotary evaporator, and the resulting material was precipitated several times with deionized water, and the product was identified by NMR spectroscopy in a yield of 4.5 g (68%). 1H-NMR (DMSO-d6): (ppm) 5.62 (s, CH, 4H), 6.16 (s, ArH, 4H), 6.21 (broad s, A...
Embodiment 2
[0089]Example 2: In this example, the 4-(1,1,1,3,3,3-hexafluoro-2-hydroxy-2-propyl)phenylresorcinolcalix[4 ]arenes were selectively functionalized with tert-butoxycarbonyl (t-BOC) at the phenolic hydroxyl unit by a base-catalyzed reaction to generate 4-(1,1,1,3,3,3-hexafluoro -2-Hydroxy-2-propyl)phenylresorcinolcalix[4]arene (structure 2 shown below).
[0090] Structure 2
[0091]
[0092] 4-(1,1,1,3,3,3-hexafluoro-2-hydroxy-2-propyl)phenylresorcinolcalix[4]arene (2 g, 0.0014 mol) and 4-bis Methylaminopyridine (0.06 g, 0.0005 mol) was dissolved in 25 mL of acetone in a round bottom flask equipped with a stirrer. Di-tert-butyl dicarbonate (2.39 g, 0.011 mol) was added slowly using a dropping funnel, followed by steady stirring. CO released immediately 2 Gas indicated the progress of the reaction, and the reaction mixture was stirred at room temperature for 8 hours. The solvent was reduced by evaporation, and the product was purified by column chromatography using aceton...
Embodiment 3
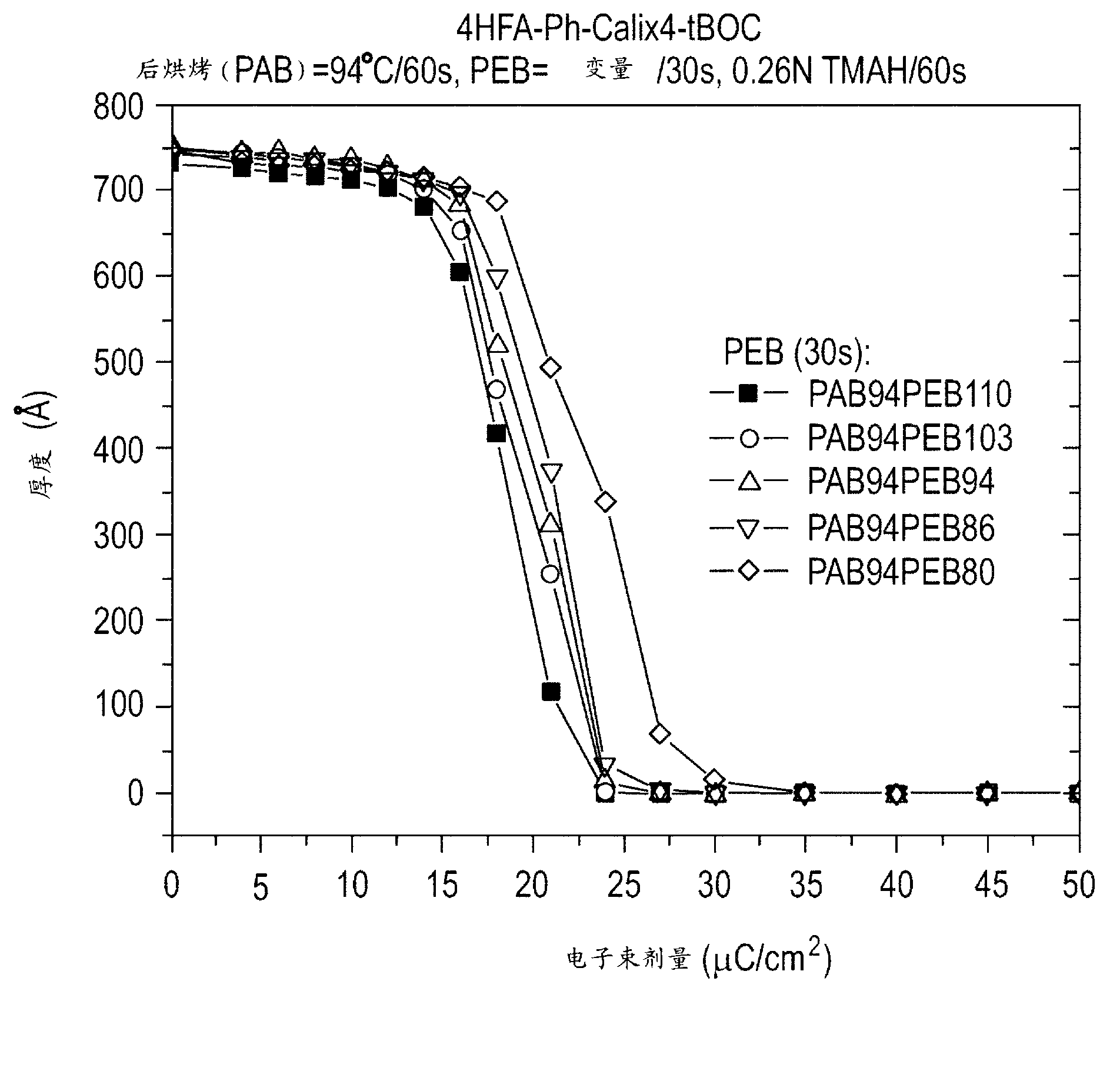
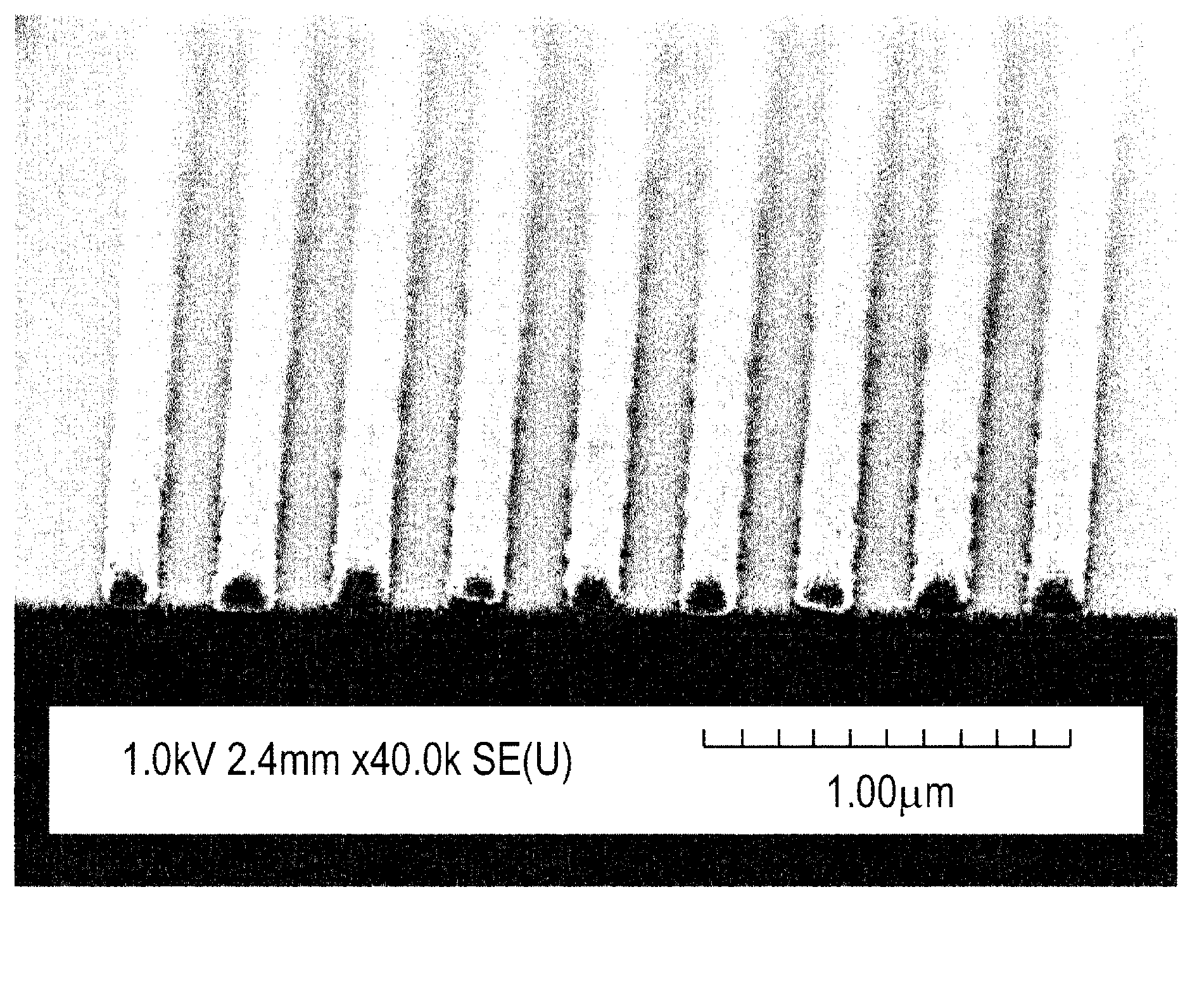
[0093] Example 3: In this example, the t-BOC-protected 4-(1,1,1,3,3,3-hexafluoro-2-hydroxy-2-propyl)benzene comprising Example 2 Lithographic evaluation of photoresist compositions based on resorcinol calix[4]arene. The photoresist was prepared as 0.25 g of t-BOC-protected 4-(1,1,1,3,3,3-hexafluoro-2-hydroxyl in 5 g of propylene glycol monomethyl ether acetate (PGMEA) -2-propyl)phenylresorcinolcalix[4]arene (structure 2), 25 mg of triphenylthioperfluoro-1-butanesulfonate (photoacid generator), and 0.75 mg of organic Alkaline preparation. The solution was filtered through a 0.2 micron syringe filter.
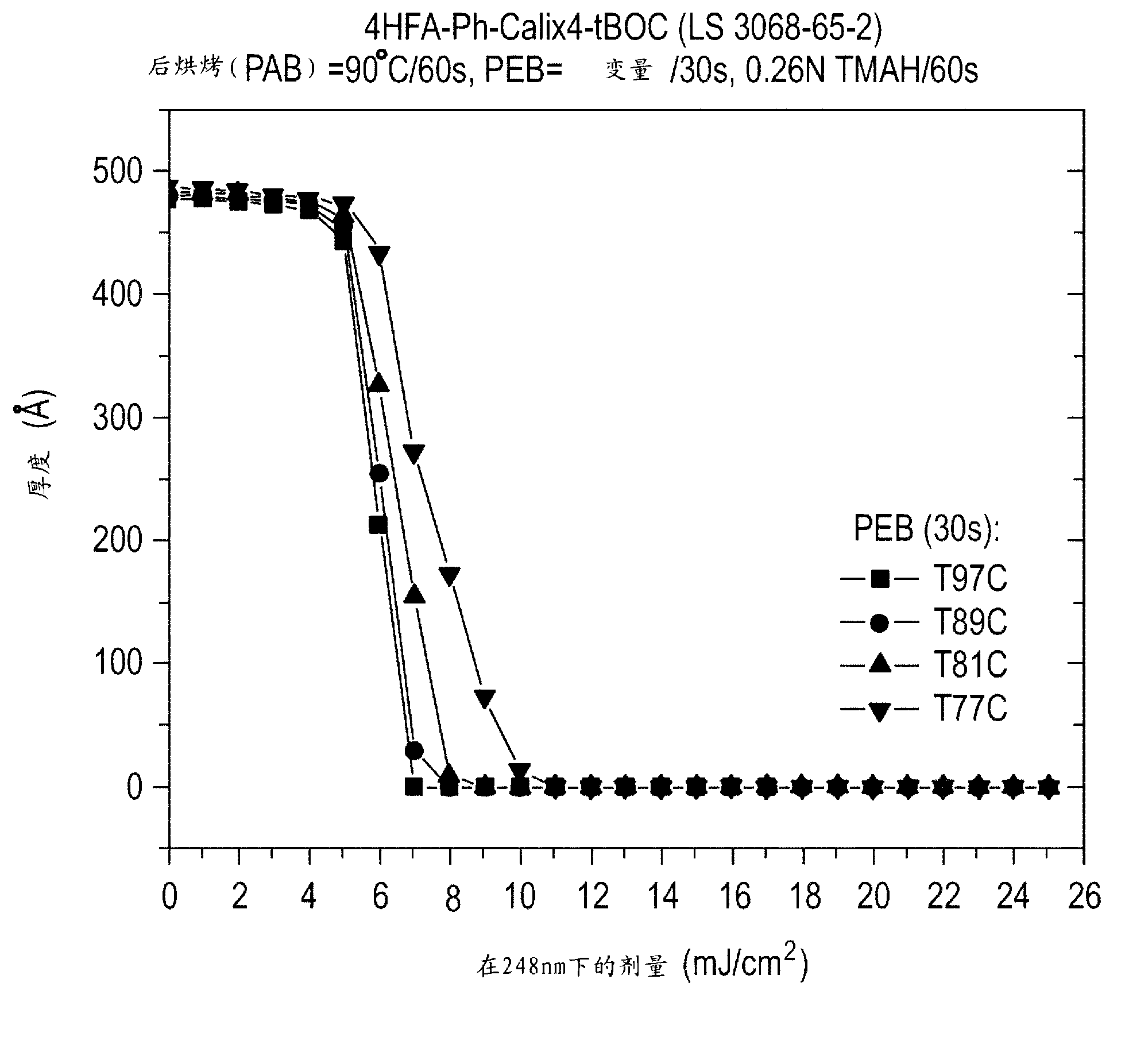
[0094] The photoresist composition was spin-coated on a blank silicon wafer, and then baked at a temperature of 90° C. for 60 seconds to form a thickness of about 500 angstroms (50 nm). figure 1 The comparative curve at 248 nm in 0.26N TMAH developer is graphically illustrated as a function of 30 second post exposure bake temperature generated using an ASML stepper. As shown,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com