Positive pole material for lithium ion battery and preparation method of positive pole material
A technology for lithium-ion batteries and positive electrode materials, applied in battery electrodes, circuits, electrical components, etc., can solve the problems of reduced discharge capacity of positive electrode materials, increased pH value, and failure to consider the impact of electrochemical performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] First weigh 4.1g of boric acid, dissolve it in 100ml of distilled water, then weigh 200g of the positive electrode material synthesized in Comparative Example 1 and add it to the prepared boric acid aqueous solution, stir fully at room temperature for 2 hours, then place it in a water bath at 100°C and stir until the solvent is dissolved Volatilize completely, and then put it into a vacuum oven and bake at 200°C for 12 hours to obtain Li 2 B 4 o 7 Coated LiNi 0.5 co 0.2 mn 0.3 o 2 Cathode material.
Embodiment 2
[0055] First weigh 1.3g of phosphoric acid, dissolve it in 100ml of distilled water, then weigh 200g of the positive electrode material synthesized in Comparative Example 1 and add it to the prepared phosphoric acid aqueous solution, stir fully at room temperature for 2 hours, then place it in a water bath at 100°C and stir until the solvent dissolves Completely volatilized; then put it into a vacuum oven and bake at 200°C for 12 hours to obtain Li 3 PO 4 Coated LiNi 0.5 co 0.2 mn 0.3 o 2 Cathode material.
Embodiment 3
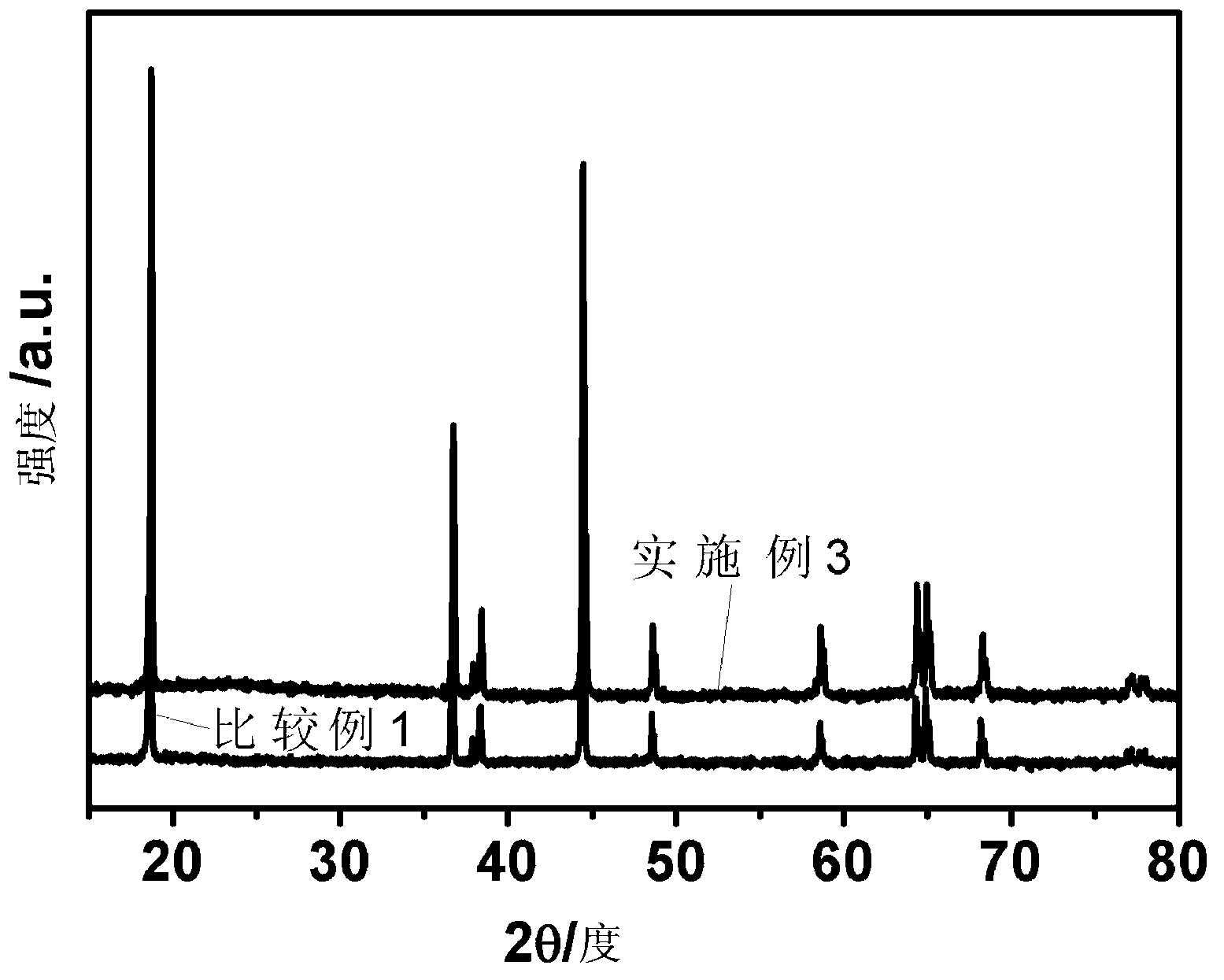
[0057] First measure 5.9ml tetrabutyl titanate (C 16 h 36 o 4 Ti), dissolved in 50ml of absolute ethanol, added 50ml of water and 2ml of acetic acid as a catalyst to accelerate the hydrolysis of tetrabutyl titanate; then, weighed 200g of the material synthesized in Comparative Example 1 and added it to the above mixture, and stirred at room temperature for 4h Finally, heat and stir in a 90°C water bath until the solvent is completely volatilized; finally, put the completely volatilized material in a vacuum drying oven at 120°C for 12 hours to obtain Li 2 TiO 3 Coated LiNi 0.5 co 0.2 mn 0.3 o 2 Cathode material.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com