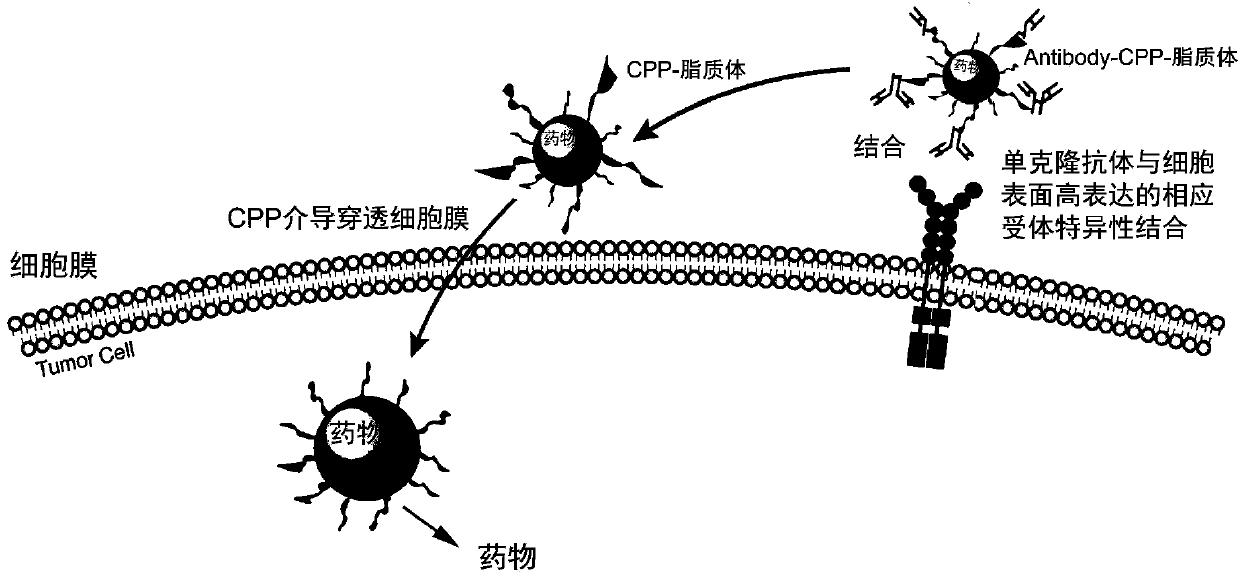
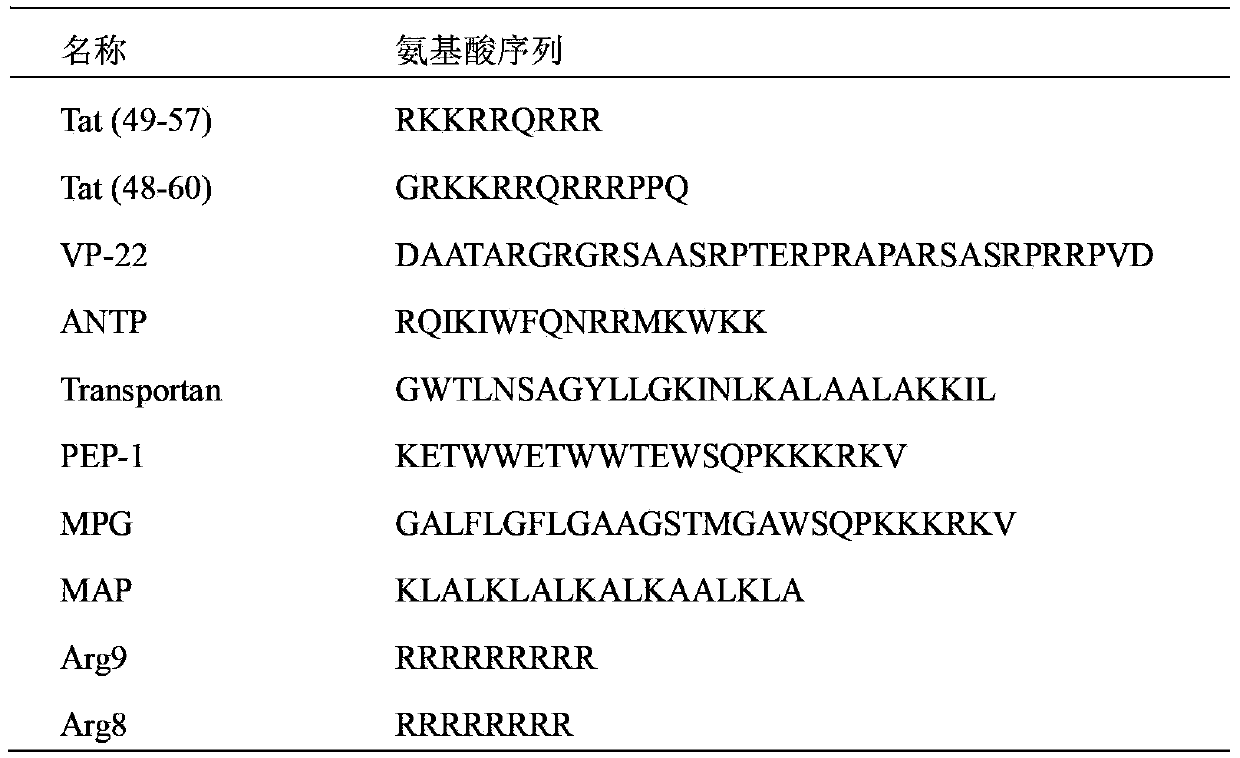
Preparation method of propranolol stealth liposome modified by vascular endothelial growth factor receptor-2 (VEGFR-2) monoclonal antibody and casein phosphopeptides (CPP) jointly, and products thereof
A stealth lipid technology of VEGFR-2 and naphthalene, applied in liposome delivery, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve the abnormal development of tissues and organs and lack of targeting Therapeutic effect, increased patient pain and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Establish the HPLC-FID analysis method of measuring body fluid propranolol concentration:
[0040] The concentration of propranolol in blood, tissue and cell culture medium was determined by HPLC-FID (fluorescence) detection method.
[0041] Specific method: Carvedilol was used as internal standard. Detection wavelength: excitation light 290nm, emission light 340nm. Chromatographic column: C18 column (250×4.6mm, 2.5μm). Add 10 microliters of internal standard solution to biological samples, add 50 microliters of saturated sodium carbonate and 2.5mL of a mixed solvent (n-hexane-dichloromethane-isopropanol=65:30:5) and vortex for 3 minutes, centrifuge at 4000rpm for 10 minutes, and separate The supernatant was blown dry with nitrogen, reconstituted in 100 μL methanol and injected 50 μL for determination.
Embodiment 2
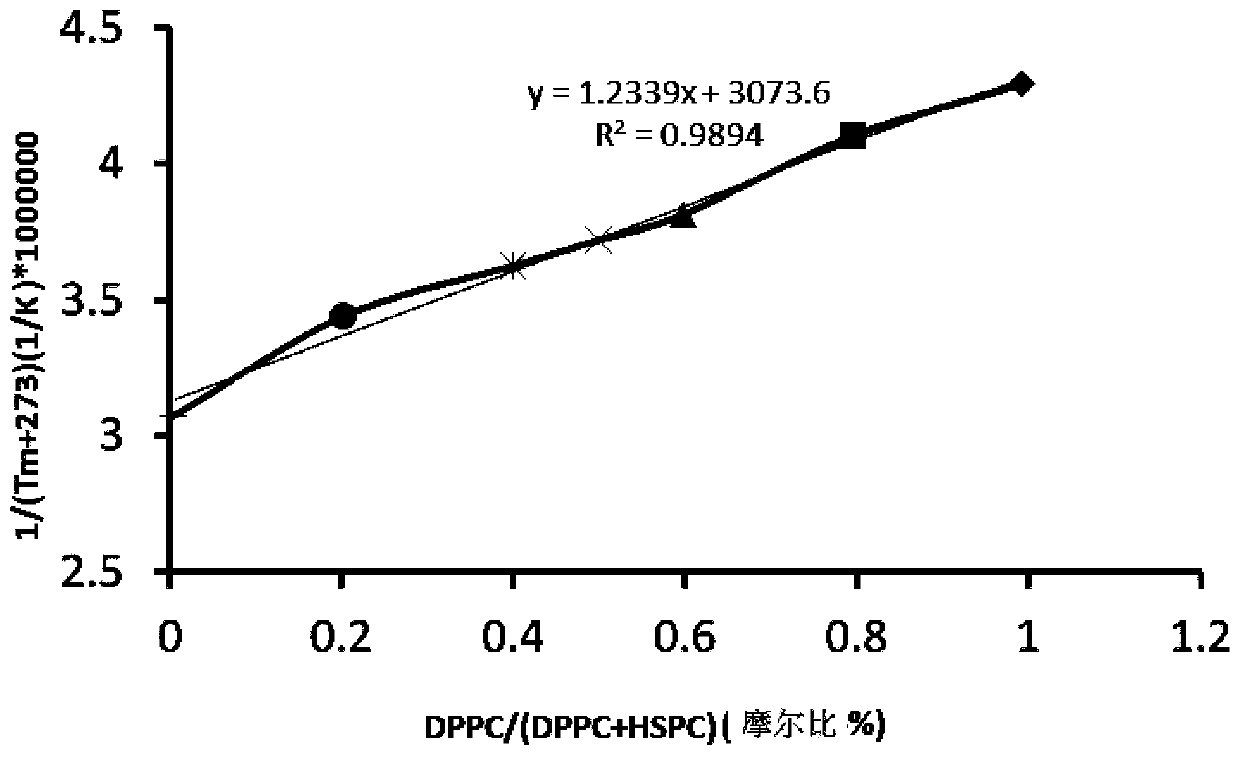
[0043] Determination of the composition and ratio of complex phospholipids: using distearoylphosphatidylcholine (DSPC) and hydrogenated soybean phosphatidylcholine (HSPC) as high phase transition temperature phospholipids; using dipalmitoylphosphatidylcholine (DPPC) and dimyristoyl Phosphatidylcholine (DMPC) is a phospholipid with a low phase transition temperature, and two combinations are used to prepare complex phospholipid liposomes (a total of 4 combinations). In the case of adding the stealth modification material DSPE-PEG2000, different combinations and different combinations under the same combination are compared. The effect of phospholipid ratio on drug loading of propranolol and its stability in plasma (that is, the leakage rate of propranolol). Finally, the composition and ratio of the complex phospholipids with good drug-loading effect and stability are determined for the following liposome research. The optimal ratio is: distearoylphosphatidylcholine (DSPC), hydr...
Embodiment 3
[0045] Preparation of complex phospholipid liposomes: prepared by thin film-ultrasonic method. Weigh 120mg of phospholipid material and dissolve in 10mL of chloroform, put it in a 50mL eggplant-shaped bottle and evaporate it under reduced pressure at 60°C to form a thin film, take out the eggplant-shaped bottle, and place it in a vacuum drying oven at 55°C for 12h. Add 5mL of ammonium sulfate to the eggplant-shaped bottle, and rotate and hydrate at 60°C for 40min. The obtained liposome suspension is ultrasonicated (400W, 50 times) in a water bath at 60°C, centrifuged at 1000rpm at low speed to remove titanium powder, and dialyzed with pH7.4PBS to remove the outer layer. Water phase ammonium sulfate, add propranolol powder and mix, 60 ℃ 20min, that is. Sephadex G-50 column (inner diameter 1cm, length 27cm) on drug-loaded liposomes, eluted with pH 7.4 PBS, flow rate 1mL min -1 , the liposome part and the free part were collected after loading on the column, and the concentratio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| clearance rate | aaaaa | aaaaa |
| encapsulation rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com