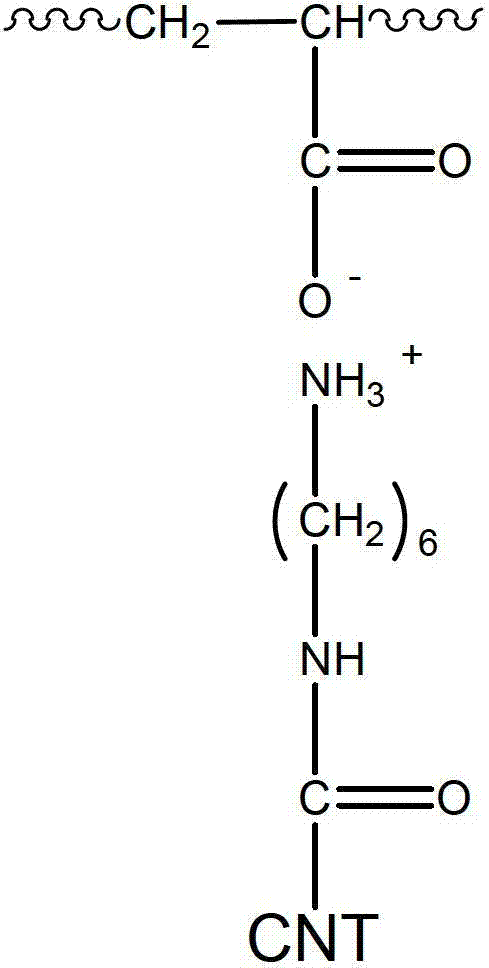Binder for electrode of lithium battery and lithium battery containing the binder
A binder and lithium battery technology, applied in battery electrodes, adhesives, secondary batteries, etc., can solve problems such as difficult to achieve the required capacity of non-carbon materials, and achieve the effect of increasing life and improving conduction paths
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0071] Preparation Example 1: Preparation of Pretreated Carbon Nanotube (CNT) Composition
[0072] (1) Introduction of carboxyl groups by acid treatment of CNT
[0073] 2 g of multi-walled CNTs having a diameter of about 10 nm to 20 nm and a length of about 10 μm to 50 μm were treated with 300 ml of a 20 wt% nitric acid solution at 40° C. for 24 hours. Then, 1 g of the cleaned CNTs was soaked in 200 ml of a 3:1 (v / v%) solution of concentrated sulfuric acid and concentrated nitric acid, treated with ultrasonic waves at room temperature for 3 hours, and then stirred at 70 °C for 6 hours. Next, the acid solution on the CNTs was removed by filtration and washed with pure water several times, and then the resulting product was dried in a vacuum oven at 80 °C for 24 hours to prepare CNTs with carboxyl groups (raw material A).
[0074] (2) Modification of CNTs with carboxyl groups (1)
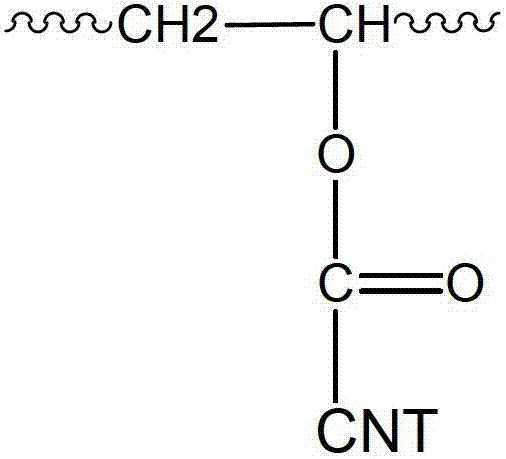
[0075] Carboxyl groups on the surface of CNTs were modified into chloroacyl groups by subj...
preparation example 2
[0080] Preparation Example 2: Preparation of Water-Based Adhesive Composition
[0081] 3 g of poly(vinyl alcohol) (PVA, saponified: about 87% to about 89%, average Mw: about 31,000 g / mol to about 50,000 g / mol) dried under vacuum at 110°C for 24 hours and 27 g of anhydrous di Methylacetamide (DMAc) was mixed in a reaction vessel, stirred and heated at 100 °C under a nitrogen atmosphere for 6 h to completely dissolve the PVA in DMAc. Then, the temperature of the reaction vessel was lowered to room temperature, and 0.2 g of calcium hydride (CaH 2 ) into a reaction vessel, stirred for 24 hours to remove the remaining moisture, and filtered to obtain a solution. 18 g of the solution and 0.2 g of material B were placed in a reaction vessel, sonicated for 10 minutes, then stirred for 1 hour. Then, 0.1 ml of purified triethylamine was injected into the reaction vessel with a syringe, and then the temperature of the reaction vessel was raised to 60° C. and the reaction vessel was s...
preparation example 3
[0085] Preparation Example 3: Preparation of Water-Based Adhesive Composition
[0086] 0.9 g of poly(acrylic acid) (PAA, average Mw: about 450,000 g / mol) dried in vacuum at 110°C for 24 hours and 19 g of pure water were mixed in a reaction vessel, stirred and heated at 60°C for 6 hours to make PAA Completely soluble in pure water. Then, the temperature of the reaction vessel was lowered to room temperature, 0.1 g of raw material C was put into the reaction vessel, treated with ultrasonic waves for 10 minutes, and then stirred for 24 hours to prepare an aqueous binder composition (solution F), in which CNTs are ionically linked to PAA. Here, the chemical structure of the binder contained in Solution F has a repeating unit shown in General Formula 2 below.
[0087] Formula 2
[0088]
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com