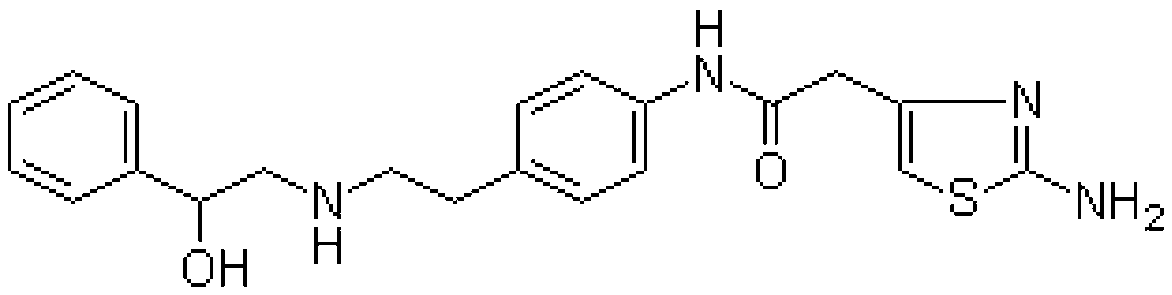
Synthesis method of mirabegron
A synthesis method and technology of mirabegron, applied in the field of chemical drug synthesis, can solve problems such as unfavorable large-scale production, cumbersome post-processing steps, difficult recovery and application, etc., and achieve convenient large-scale industrial production and simple and easy post-reaction treatment. , the effect of strong market competitiveness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
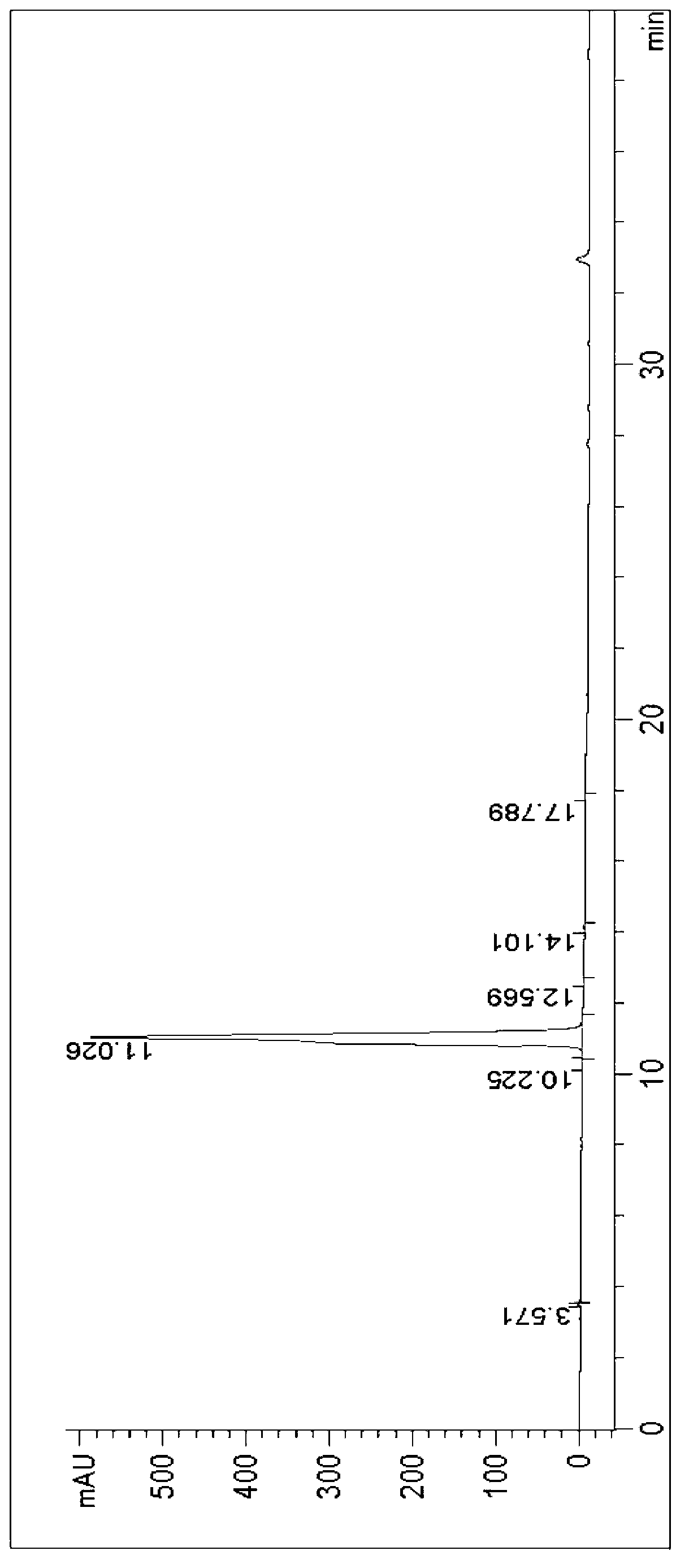
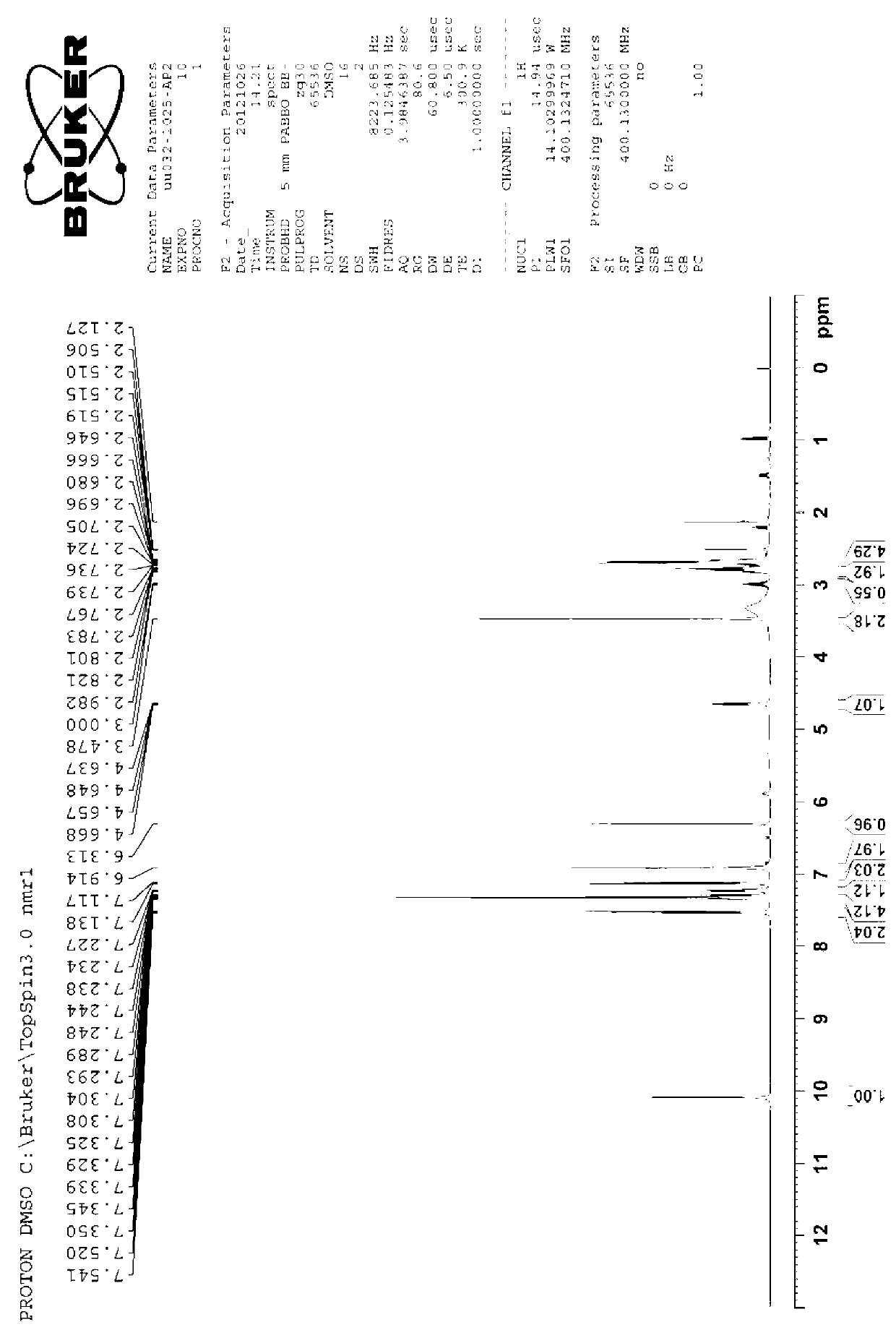
Image
Examples
Embodiment 1
[0066] At 0°C, 1.59g of 2-aminothiazole-5-acetic acid was dissolved in 50mL of tetrahydrofuran to form a reaction solution, and 3.27g of (Boc) 2 O was added dropwise to the reaction solution. The reaction solution was raised to room temperature and stirred for 12 hours to make 2-aminothiazole-5-acetic acid and (Boc) 2 O response. After the completion of the reaction detected by TLC, the reaction solution was concentrated to obtain a crude product, which was recrystallized with ethanol / water to obtain 2.5 g of Mirabegron intermediate product A.
[0067] Add 5.16g of Mirabegron intermediate A to a 100mL three-necked flask and dissolve in 20mL of N,N-dimethylformamide, and stir at 20°C until uniformly mixed to form a mixed solution. Add 3.24g of N,N'-carbonyldiimidazole into a conical flask and dissolve in 10mL of N,N-dimethylformamide, fully dissolve at 30°C and cool to 20°C, then add dropwise to the above mixed solution, drop Stir for 1.5 minutes until TLC and HPLC show comp...
Embodiment 2
[0071] Under the condition of 0°C, 3.16g of 2-aminothiazole-5-acetic acid was dissolved in 100mL of methanol to form a reaction solution, and 3.375g of benzyloxycarbonyl amino protecting agent was added dropwise into the reaction solution while stirring. The reaction solution was raised to room temperature and stirred for 12 hours to react 2-aminothiazole-5-acetic acid with benzyloxycarbonyl amino protecting agent. After the completion of the reaction detected by TLC, the reaction solution was concentrated to obtain a crude product, which was recrystallized with ethanol / water to obtain 5.67 g of Mirabegron intermediate product A.
[0072] Add 5.67g of Mirabegron intermediate A to a 100mL three-necked flask and dissolve in 20mL of dichloromethane, and stir at 22°C until uniformly mixed to form a mixed solution. Add 2.97g of N,N'-carbonyldiimidazole into a conical flask and dissolve in 10mL of dichloromethane, fully dissolve at 30°C and cool to 22°C, then add dropwise to the abo...
Embodiment 3
[0076] Under the condition of 0°C, 2.37g of 2-aminothiazole-5-acetic acid was dissolved in 80mL of tert-butanol to form a reaction solution, and 5.49g of trityl amino protecting agent was added dropwise into the reaction solution while stirring. The reaction solution was raised to room temperature and stirred for 12 hours to react 2-aminothiazole-5-acetic acid with trityl amino protecting agent. After the reaction was detected by TLC, the reaction solution was concentrated to obtain a crude product, which was recrystallized with ethanol / water to obtain 8.9 g of mirabegron intermediate product A.
[0077] Add 8.9g of Mirabegron intermediate A to a 100mL three-neck flask and dissolve in 20mL of acetonitrile, and stir at 25°C until uniformly mixed to form a mixed solution. Add 5.5g of N,N'-diisopropylcarbodiimide into a conical flask and dissolve it in 10mL of acetonitrile, fully dissolve at 30°C and cool to 25°C, then add dropwise to the above mixed solution, after the drop Stir ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com