Method for preparing ortho-hydroxybenzoic acid by catalyzing and oxidizing ortho-nitrotoluene with metalloporphyrin and metal salt compound as catalyst
A technology of o-nitrobenzoic acid and composite catalyst, which is applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve the problems of methanol and benzene solvents with high toxicity, increased energy consumption, environmental pollution, and difficulty in wastewater treatment. problems, to achieve the effect of shortening the reaction time, reducing environmental pollution, and reducing the amount of acid.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
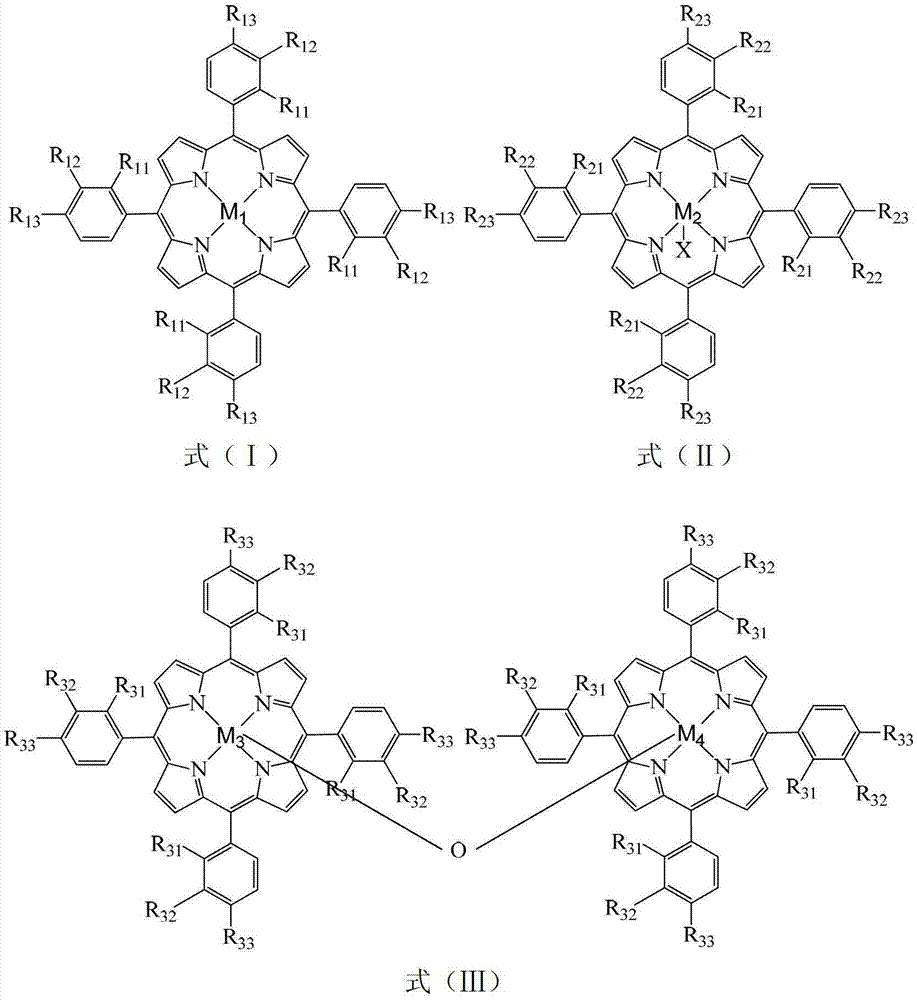
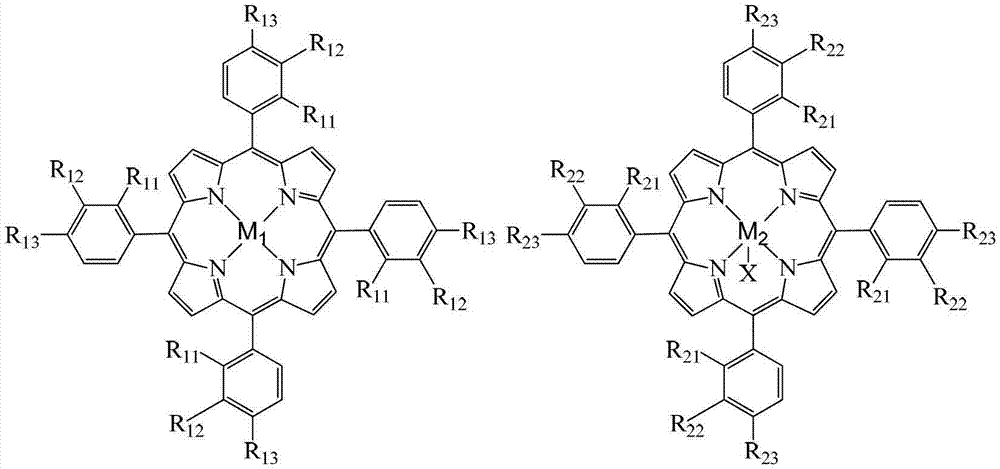
[0021] Take 8.6×10 -5 g chloride tetra-(p-chlorophenyl) cobalt porphyrin (i.e. R in the general formula (II) 21 =R 22 = H, R 23 =Cl,X=Cl,M 2 =Co), 1.8×10 -5 g manganese acetate, 1.4 g o-nitrotoluene and 2.0 g sodium hydroxide are added to a 100 mL autoclave, 20 mL of ethanol aqueous solution containing 80% (V:V) of ethanol is added, and oxygen at a pressure of 2.0 MPa is introduced, and in a water bath The temperature was controlled at 55°C for 6 hours. After the reaction was completed, the reaction liquid was detected by high performance liquid chromatography, and the conversion rate of o-nitrotoluene was 98.2%, the selectivity of o-nitrobenzoic acid was 97.9%, and the yield of o-nitrobenzoic acid was 96.1%.
Embodiment 2
[0023] Take 8.6×10 -5 g chloride tetra-(p-nitrophenyl)iron porphyrin (i.e. R in the general formula (II) 21 =R 22 = H, R 23 =NO 2 , X=Cl,M 2 =Fe), 3.8×10 -5 1g zinc chloride, 1.6g o-nitrotoluene and 1.8g sodium hydroxide are added to a 100mL autoclave, 20mL of ethanol aqueous solution containing 90% (V:V) of ethanol is added, and the pressure is 1.8MPa of oxygen, and the The reaction was carried out at a temperature of 50° C. for 5 hours. After the reaction was completed, the reaction liquid was detected by high performance liquid chromatography, and the conversion rate of o-nitrotoluene was 93.0%, the selectivity of o-nitrobenzoic acid was 96.2%, and the yield of o-nitrobenzoic acid was 89.5%.
Embodiment 3
[0025] Take 1.7×10 -4 g four-(p-chlorophenyl) manganese porphyrin (i.e. R in the general formula (I) 11 =R 12 = H, R 13 =Cl,M 1 =Mn), 9.5×10 -5 g cobalt acetate, 1.0 g o-nitrotoluene and 1.5 g sodium hydroxide are added to a 100 mL autoclave, 20 mL of ethanol aqueous solution containing 70% (V:V) of ethanol is added, and oxygen at a pressure of 1.5 MPa is introduced, and in a water bath The temperature was controlled at 45°C for 4 hours. After the reaction was completed, the reaction liquid was detected by high performance liquid chromatography, and the conversion rate of o-nitrotoluene was 76.2%, the selectivity of o-nitrobenzoic acid was 95.1%, and the yield of o-nitrobenzoic acid was 72.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com