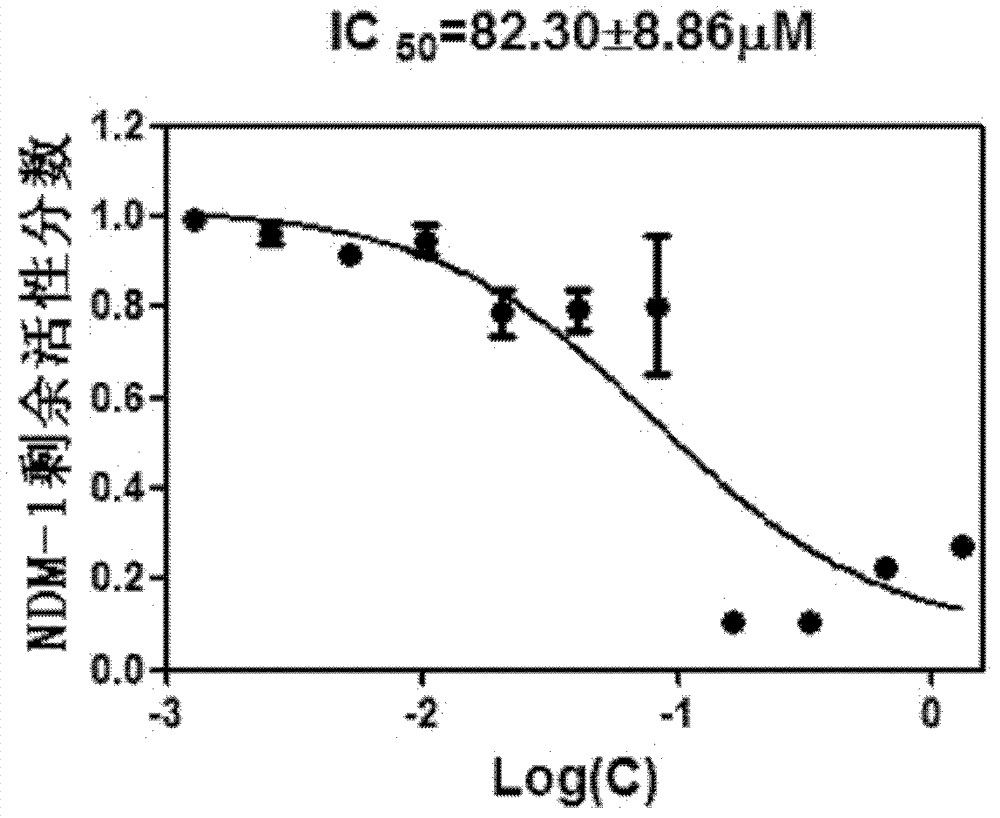
Application of sulfonamide compounds in inhibiting NDM-1 activity
A technology of sulfonamides and compounds, applied in the field of medicinal chemistry, can solve problems such as high in vitro sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 14
[0055] The preparation of embodiment 14-iodo-N-(3-methoxyphenyl) benzenesulfonamide
[0056]
[0057] Select a 50ml round-bottomed flask as the reaction vessel, and at 0°C, dissolve p-iodobenzenesulfonyl chloride (302mg, 1mmol) in 10ml of ethanol, drop the solution into m-methoxyaniline (123mg, 1mmol), and then add Sodium bicarbonate (170mg, 2mmol), it is moved to room temperature, electromagnetic stirring, after TLC detects that reaction is complete, stops reaction, and 10ml water is joined in the solution after reaction, and white solid appears, and it is filtered out, to The obtained white solid was vacuum-dried to obtain 320 mg of the title compound with a yield of 82%. The identification data of this compound are as follows: ESI-MS: m / z 390.20 ([M+H + ]); 1 HNMR (400MHz, CDCl 3 , δppm): 7.82(dd, J=2.0Hz, 2.8Hz, 2H), 7.49(dd, J=2.0, 2.8Hz, 2H), 7.16(t, J=8.4Hz, 1H), 6.71(m, 2H ), 6.61 (d, J=1.2Hz, 1H), 6.51 (s, 1H), 3.78 (s, 3H).
Embodiment 24
[0058] The preparation of embodiment 24-methyl-N-phenylbenzenesulfonamide
[0059]
[0060] Replace the p-iodobenzenesulfonyl chloride in Example 1 with p-toluenesulfonyl chloride, and replace the m-methoxyaniline in Example 1 with aniline as a starting material, and use steps similar to Example 1 to prepare the compound , the yield was 76%. This compound is a white solid, and its identification data are as follows: ESI-MS: m / z 248.21 ([M+H + ]); 1 HNMR (400MHz, DMSO-d 6 , δppm): 10.19(s, 1H), 7.63(d, J=8.4Hz, 2H), 7.33(d, J=4.0Hz, 2H), 7.29(d, J=7.8Hz, 1H), 7.06(m , J=7.0Hz, 1H), 6.14(s, 1H), 2.49(s, 3H).
Embodiment 34
[0061] The preparation of embodiment 34-methyl-N-naphthylbenzenesulfonamide
[0062]
[0063] Replace the p-iodobenzenesulfonyl chloride in Example 1 with p-toluenesulfonyl chloride, and replace the m-methoxyaniline in Example 1 with 1-naphthylamine as a starting material, using steps similar to Example 1 , the compound was prepared with a yield of 72%. The compound is a white solid, and its identification data are as follows: ESI-MS: m / z 298.21 ([M+H + ]); 1 HNMR (400MHz, DMSO-d 6 , δppm): 10.16(s, 1H), 8.04(d, J=8.4Hz, 1H), 7.88(d, J=8.4Hz, 1H), 7.77(d, J=8.4Hz, 1H), 7.58(d , J=8.0Hz, 2H), 7.38(m, 3H), 7.30(d, J=8.0Hz, 2H), 7.14(dd, J=0.8, 7.6Hz, 1H), 2.33(s, 1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com