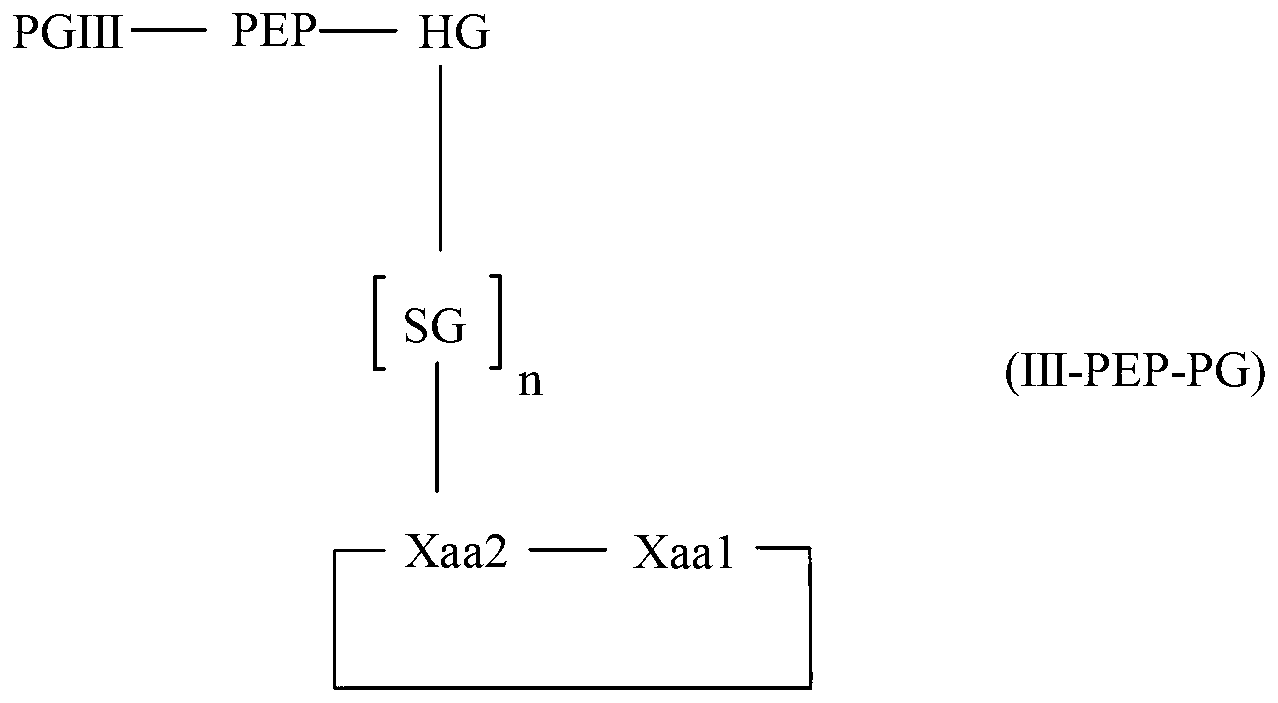
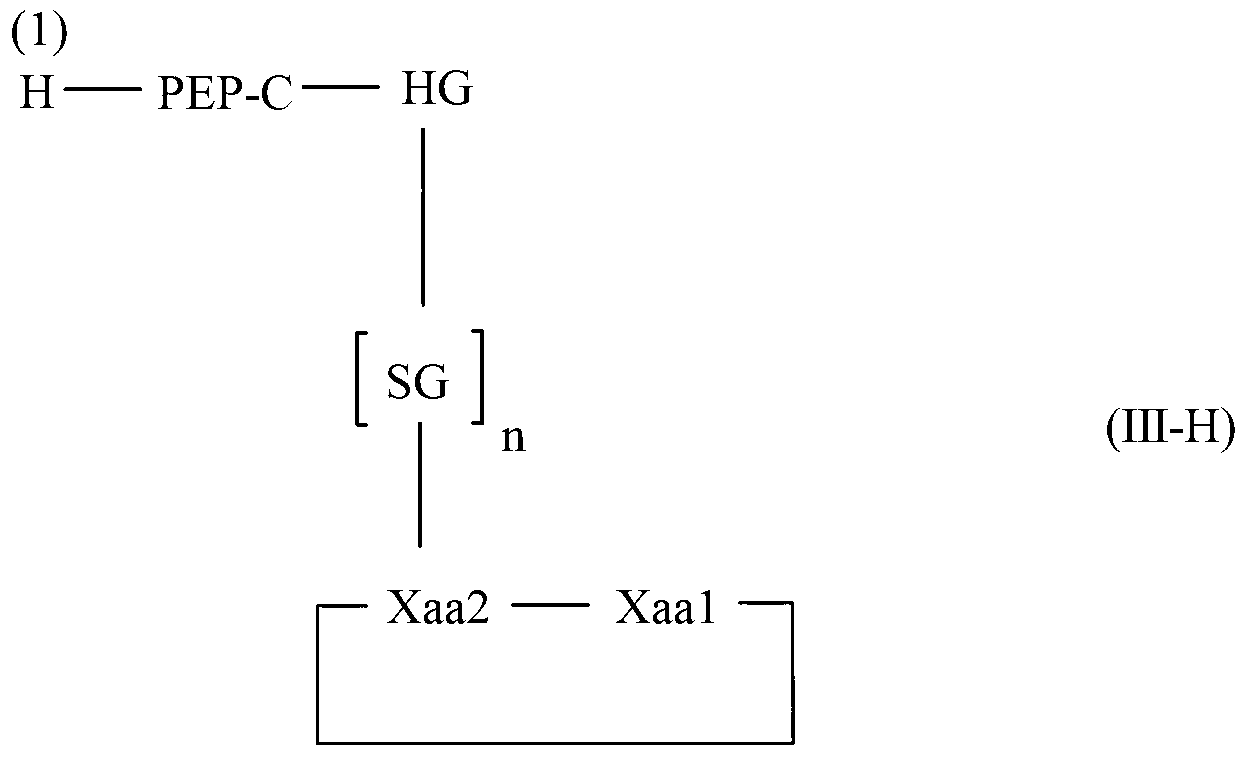
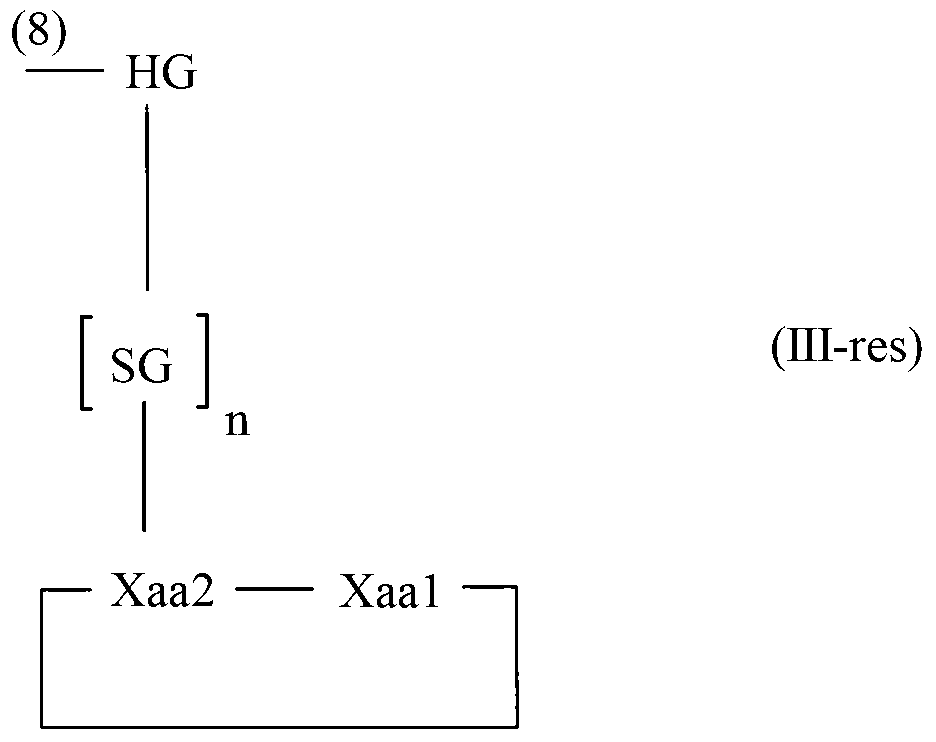
Diketopiperazine forming dipeptidyl linker
A kind of residue, resin technology, applied in the field of dipeptide linker forming diketopiperazine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[1197] Example 1: SPPS (0.1 mmol scale) using T20C linked to resin via a dipeptide linker forming a diketopiperazinyl group
[1198] Manual SPPS.
[1199] Method Fmoc-gr-rem (removal of Fmoc group)
[1200] In the following examples, the Fmoc group was removed by these procedures:
[1201] Use 20% (v / v) piperidine in DMF (1 x 1 min, 2 x 10 min; 3 ml each for Examples 1 to 3 and 7, 150 ml each for Examples 4 to 6) Process the resin, followed by:
[1202] DMF washes (5 x 1 min; 3 ml each) (for Examples 1 to 3 and Example 7),
[1203] Continue with DMF wash (600ml) and Chloranil Test Method D (for Examples 4 to 6).
Embodiment 11
[1204] Example 1.1 Attachment of a dipeptide linker forming a diketopiperazinyl group to a resin
[1205] a) Pretreatment of HMPS resin
[1206] HMPS resin (106.2 mg) was swelled with DCM (5 x 1 min; 3 ml each) and DMF (5 x 1 min; 3 ml each) at room temperature and then filtered.
[1207] b) Incorporation of the first amino acid (D-Pro) of the dipeptide linker forming the diketopiperazinyl group onto the resin
[1208] Fmoc-D-Pro-OH (135 mg, 4 equiv) and DIPCDI (31 μl, 2 equiv) in DCM / DMF (15:1, v / v, 2.5 ml) were added to in the prepared resin. Next, DMAP (4.9 mg, 0.4 equiv) in DCM (0.5 ml) was added and left at room temperature for 2 hours. Fmoc-D-Pro-OH was incorporated again at the end of this procedure. After the previous 2 hour coupling time, the resin was washed with DCM (5 x 1 min; 3 ml each) and with DMF (5 x 1 min; 3 ml each). The resin was then capped with acetic anhydride (47 μL, 5 eq) and DIEA (85 μL, 5 eq) in DMF (2.5 ml) for 30 min at room temperature. Afte...
Embodiment 12
[1214] Example 1.2 Synthesis of T20C by SPPS
[1215] Each amino acid is reacted in a reaction cycle. Follow the reaction cycle description (i) or (ii) to carry out the reaction steps in a reaction cycle to incorporate an amino acid.
[1216] a) Reaction cycle description (i)
[1217] A mixture of Fmoc-Xaa-OH (3 equiv), HCTU (140 mg, 3 equiv) and DIEA (110 μl, 6 equiv) in DMF (2 ml) was shaken for 30 seconds at room temperature, then added to the in the resin prepared in the previous step. The mixture was left standing at room temperature for 1 hour. According to the ninhydrin test (method C), no further coupling is required. The resin was then washed with DMF (5 x 1 min; 3 ml each) and with DCM (5 x 1 min; 3 ml each). The Fmoc group was then removed by the method Fmoc-gr-rem.
[1218] b) Reaction cycle description (ii)
[1219] A mixture of Fmoc-Xaa-OH (3 equiv), HCTU (140 mg, 3 equiv), DIEA (110 μl, 6 equiv) in DMF (2 ml) was shaken for 30 seconds at room temperature,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com