O-phenanthroline triazole rare earth complex and preparation method thereof
A technology of phenanthroline triazoles and phenanthroline triazoles, which is applied in the field of new-type o-phenanthroline triazole rare earth complexes and their preparation, achieving the effects of cost reduction, high yield, and high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
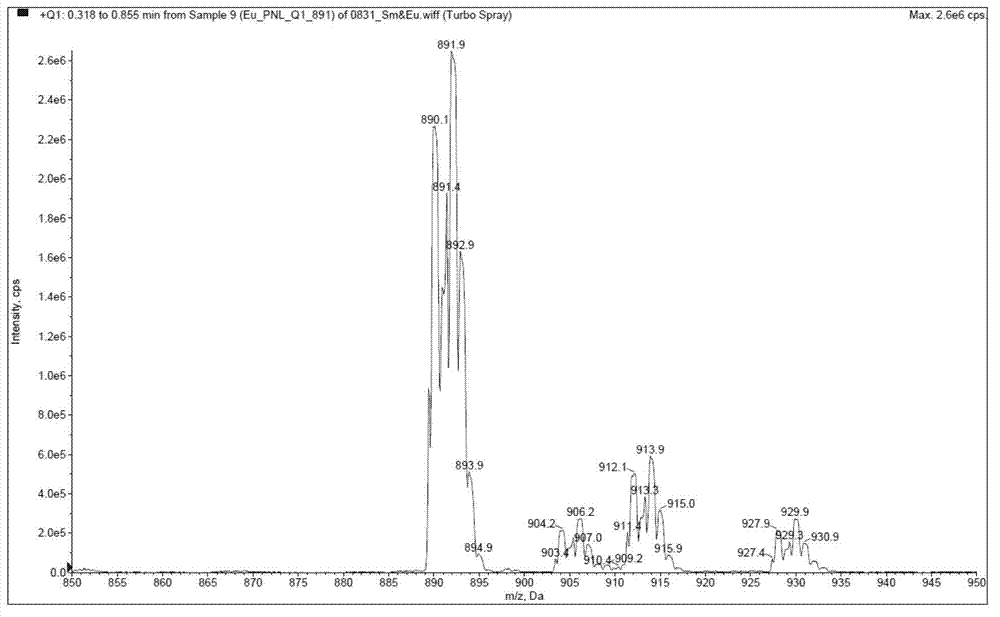
[0040] Example 1: Synthesis and fluorescence emission spectrum test of tris[5-(1,10-phenanthroline-2-yl)-1,2,4-1H-triazole]europium(III) (compound 6)
[0041] The first step: preparation of N-oxy-1,10-phenanthroline (compound 2)
[0042]
[0043] First, compound (1) o-phenanthroline (100g) and acetic acid (100mL) were added into a 2L three-neck flask, stirred evenly, and 30% H 2 o 2 Aqueous solution (75mL), heated to 70-75°C, stirred for 3 hours. Cool to room temperature, add 30% H 2 o 2 (70mL), continue heating to 60-110°C, and react for 3 hours. After cooling to room temperature, the acetic acid was concentrated under reduced pressure to obtain a reddish-brown viscous oil, which was diluted with water (500 mL), and the pH was adjusted to 9-10 with solid sodium carbonate. The resulting solution was extracted with dichloromethane (1000 mL+500 mL×3), and the organic phases were combined and dried with anhydrous sulfuric acid. After filtration, the filtrate was concentr...
Embodiment 2
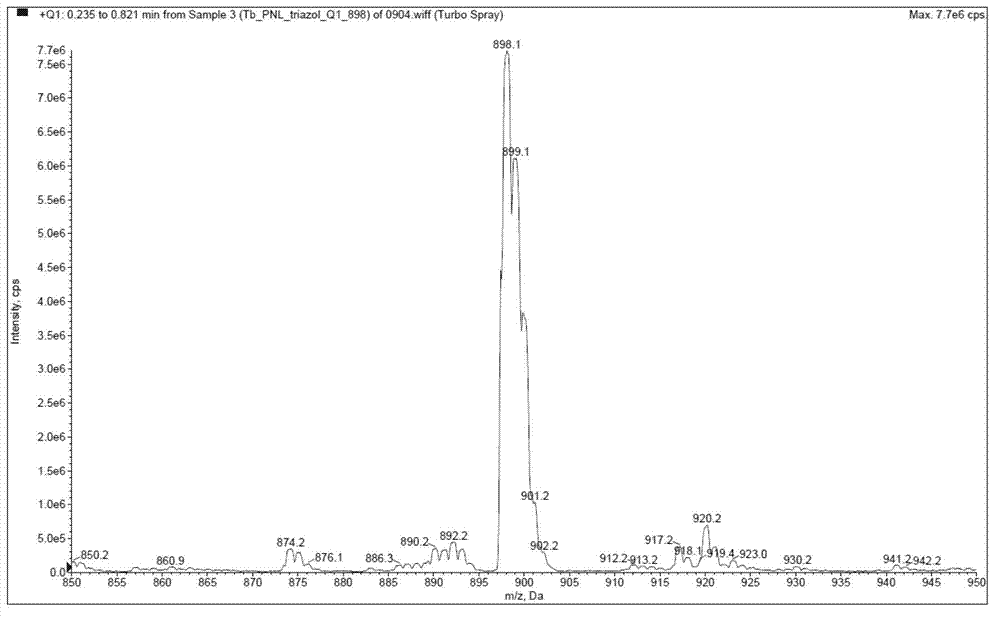
[0058] Example 2: Synthesis and fluorescence emission spectrum test of tris[5-(1,10-phenanthroline-2-yl)-1,2,4-1H-triazole]terbium(III) (compound 7)
[0059]
[0060] Compound 4 (7.4g) obtained in Example 1 and terbium trichloride hexahydrate (3.7g) were respectively dissolved in 50mL of absolute ethanol: water (V:V) = 1:3 mixed solvent to form a solution C and D. Add 1.2g of sodium hydroxide to solution C, and stir for half an hour. Then, the D solution was added dropwise into the reaction bottle of the C solution, and the reaction was stirred at room temperature for 8 hours. After the reaction, the solvent was evaporated to dryness under reduced pressure, and the solid was vacuum-dried at 50° C. for 3 hours to obtain 9.3 g of yellow powder.
[0061] Mass spectrum such as figure 2 As shown, [M+1]898.1, TbC 42 h 24 N 15 M.W.=897, the ratio of M+H peak 898 to 899 peak height is detected close to the isotopic abundance ratio of Tb 2:1.
[0062] It can be known from th...
Embodiment 3
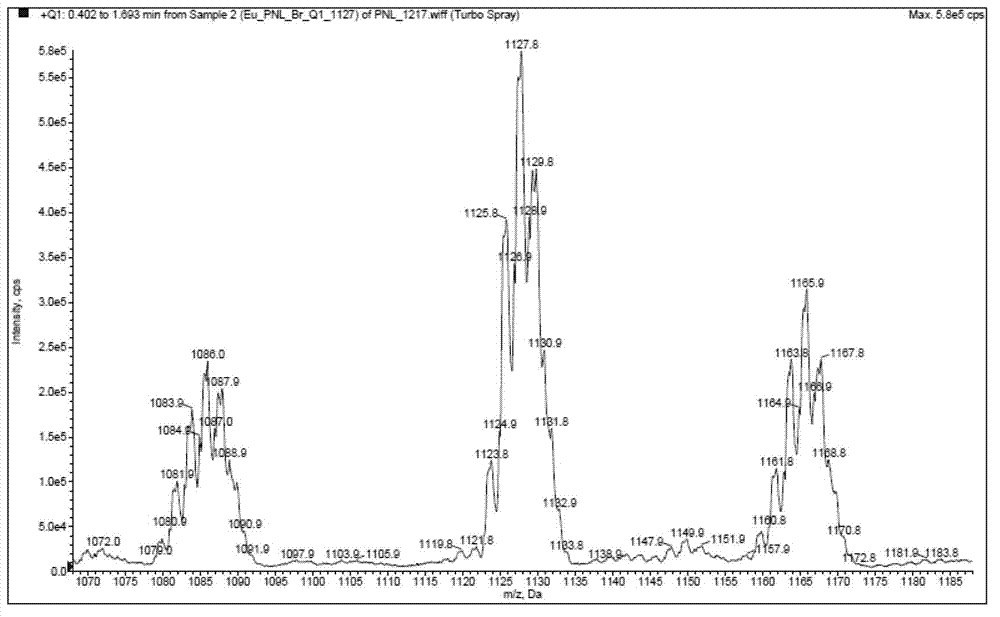
[0063] Example 3: Tris[5-(4,7-dimethyl-1,10-phenanthroline-2-yl)-1,2,4-1H-triazole]europium(III) (compound 13 ) synthesis and fluorescence emission spectrum test
[0064] Step 1: Preparation of N-oxy-4,7-dimethyl-1,10-phenanthroline (compound 9)
[0065]
[0066] First, add 4,7-dimethyl-1,10-phenanthroline (compound 8, 100g) and chloroform (800mL) into a 2L three-necked flask, stir evenly and cool to 0°C, slowly add A solution of m-chloroperoxybenzoic acid (90 g) in chloroform (600 mL). Gradually rise to room temperature, stir and react for 3 hours; continue heating to 60°C, and react for another 3 hours. Cool to room temperature, concentrate the chloroform under reduced pressure, dilute with water (1000 mL), and adjust the pH to 8-9 with solid sodium carbonate. The mixture can be heated to 90-95°C for half an hour, cooled and filtered with suction to remove unreacted raw material 4,7-dimethyl-1,10-phenanthroline. The obtained filtrate was extracted with dichloromethane...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com