Polyethylene glycol derivatives of antitumor small peptides FpAT
A technology of polyethylene glycol and acetyl polyethylene glycol, which is applied in the field of PEGylated derivatives and can solve the problems of short half-life of small peptides
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] Example 1 Cys(mPEG 2000 -MAL)-FpAT Preparation
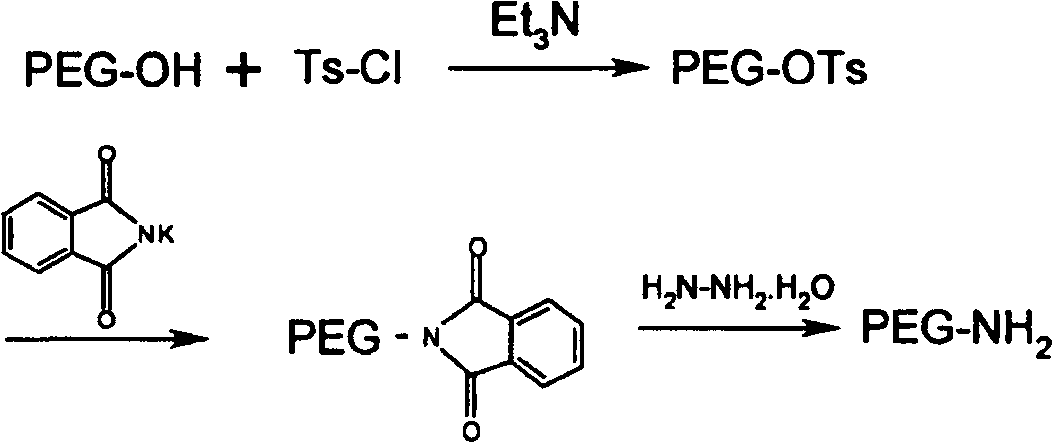
[0068] Weigh mPEG 2000 -OH 20g (10mmol) was placed in a reaction flask, dichloromethane 50mL was added, and the solid was dissolved, then triethylamine 7.5mL (50mmol) and p-toluenesulfonyl chloride 9.5g (Ts-Cl, 50mmol) were added, and the reaction was stirred at room temperature. After the reaction is complete as detected by TLC, remove the solvent by rotary evaporation, add 50 mL of anhydrous ether to precipitate a solid, add water to dissolve the solid, separate the liquid, separate the water layer, wash twice with 50 mL of anhydrous ether, and then wash the water phase with 75 mL of dichloromethane Extracted twice, collected the organic phase and dried overnight. The solvent was removed by rotary evaporation, and anhydrous diethyl ether was added to precipitate a solid. mPEG after drying 2000 -OTs 15.4g, yield 77%.
[0069] Dissolve mPEG-OTs 14g (7mmol) in 30mL DMF, add phthalimide potassium salt 3.88g (21mmol), a...
Embodiment 2
[0075] Example 2 Preparation of Cys(PEG5000-MAL)-FpAT
[0076] Weigh mPEG 5000 -OH 25g (5mmol) was placed in a reaction flask, dichloromethane 100mL was added, and the solid was dissolved, then triethylamine 7.5mL (5mmol) and p-toluenesulfonyl chloride 9.5g (Ts-Cl, 50mmol) were added, and the reaction was stirred at room temperature. After the reaction is complete as detected by TLC, remove the solvent by rotary evaporation, add 150 mL of anhydrous ether to precipitate a solid, add water to dissolve the solid, separate the liquids, separate the water layer, wash twice with 100 mL of anhydrous ether, and then wash the water phase with 150 mL of dichloromethane Extracted twice, collected the organic phase and dried overnight. The solvent was removed by rotary evaporation, the solid was precipitated by adding anhydrous ether, filtered, and dried to obtain mPEG 5000 -OTs 21.6g, yield 86%.
[0077] mPEG 5000 -OTs 20g (4mmol) was dissolved in 30mL DMF, phthalimide potassium salt...
Embodiment 3
[0081] Example 3 FpAT-Cys(PEG 2000 -MAL) preparation
[0082] Weigh 2-CTC Resin (10mmol) into the reactor, add an appropriate amount of DMF to wash, remove the solution, add an appropriate amount of DCM to swell for about 30min. Weigh 9.6g of Fmoc-Cys(Trt)-OH into a beaker, add an appropriate amount of DMF to dissolve, add 2.8mL of DIPEA to the above amino acid solution, stir evenly, and bathe in ice water for 10-15min. After the resin swells, remove the DCM, pour the dissolved protected amino acid solution into the reactor, react for 10-15 minutes, add DIPEA 5.7mL, and react at room temperature for 3-4 hours. After the reaction, remove the reaction solution, add an appropriate amount of DMF to wash 3 times, add a mixed solution of DCM, methanol, and DIPEA with a volume ratio of 17:2:1 to block unreacted groups, and block twice, about 20 min each time. . After blocking, add an appropriate volume of DMF to wash 3 times, and then wash and shrink twice with anhydrous methanol,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com