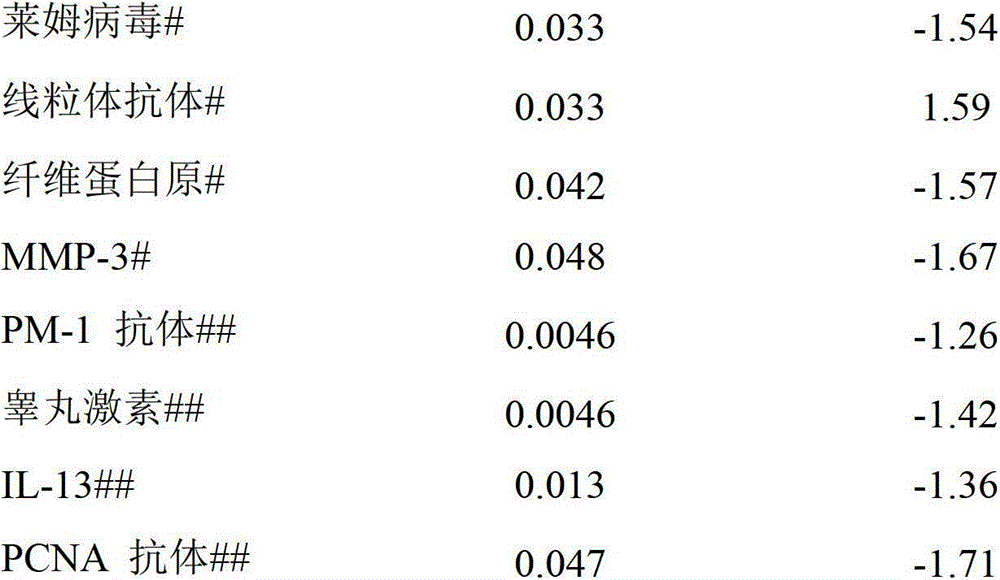Application of biomarker for preparing medicine for predicting feedback response of 5-aminosalicylic acid in treatment of ulcerative colitis
An ulcerative colitis, biomarker technology, applied in the field of biomedicine, can solve the problems of no feedback response, unclear pathophysiology and other factors, and achieve the effect of disease control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] The present invention provides sex-dependent serum target biomarkers. When a patient uses one or more biomarkers to show a level different from the normal level, it can be predicted that 5-ASA will be used to treat different colon sites (left hemicolitis, Proctosigmoid colitis, pancolitis and extensive colitis) in patients with mild to moderate UC.
[0058] 5-ASA does not respond to the application of serum biomarkers in personalized clinical practice
[0059] As mentioned above, 60% of clinical failures are encountered when using 5-ASA to treat patients with moderate UC, and 80% of clinical failures are encountered when using placebo. Due to a lack of understanding of the pathophysiology of the disease, 5-ASA therapy does not take into account differences in gender and the location of the lesion in the colon. Personalized drug treatment based on the development of clinical biomarkers will provide more effective treatment options that take into account the individual. In t...
Embodiment 2
[0070] Used to predict 5-ASA response in female patients with left hemicolitis. First determine a patient who has been diagnosed with left hemicolitis, and then confirm the diagnosis of left hemicolitis by routine colonoscopy. Usually, 5-ASA is prescribed to patients as a routine drug in the treatment of left hemicolitis. Before treating the patient’s colitis, the clinician will check one or more blood samples and obtain the samples, then prepare the serum, and finally use the above-mentioned appropriate 5-ASA non-responsive target biomarkers to determine its correlation s level. The measured target biomarker level is compared with the standard value shown in Table 2, and the result is the prediction of the efficacy of 5-ASA (the dose is 2.4 g 5-ASA / day and 4.8 g 5-ASA / day , The efficacy of treating female patients with left hemicolitis in a 6-week course of treatment). For example, if the patient's specific 5-ASA does not respond to the target biomarker and the level of the...
Embodiment 3
[0077] Methods and systems for predicting 5-ASA response in male patients with left hemicolitis. First determine a patient who has been diagnosed with left hemicolitis, and then confirm the diagnosis of left hemicolitis by routine colonoscopy. Usually, 5-ASA is prescribed to patients as a routine drug in the treatment of left hemicolitis. Before treating the patient’s colitis, the clinician will check one or more blood samples and obtain the samples, then prepare the serum, and finally use the above-mentioned appropriate 5-ASA non-responsive target biomarkers to determine its correlation s level. The measured target biomarker level is compared with the standard value shown in Table 3, and the result is the prediction of the efficacy of 5-ASA (the dose is 2.4 g 5-ASA / day and 4.8 g 5-ASA / day , The efficacy of treating male patients with left hemicolitis in a 6-week course of treatment). For example, if the patient's specific 5-ASA does not respond to the target biomarker and t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com