Omeprazole enteric capsule and prepared method thereof
A technology of omeprazole enteric and omeprazole pellets, which is applied in the field of omeprazole enteric-coated capsules and its preparation, can solve the problems of poor stability, insufficient and timely release of omeprazole enteric-coated capsules, etc. To achieve the effect of guaranteed dissolution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
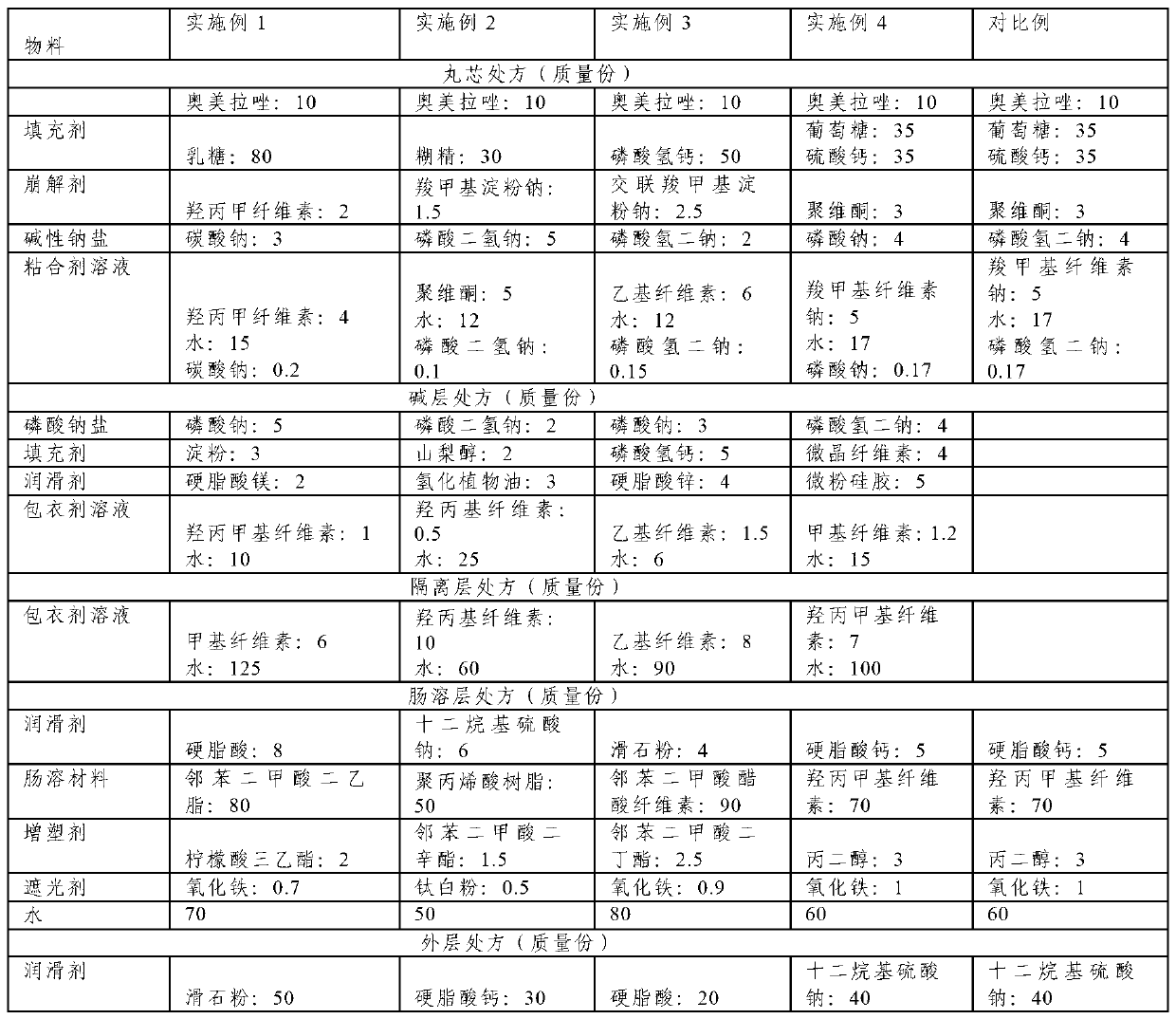
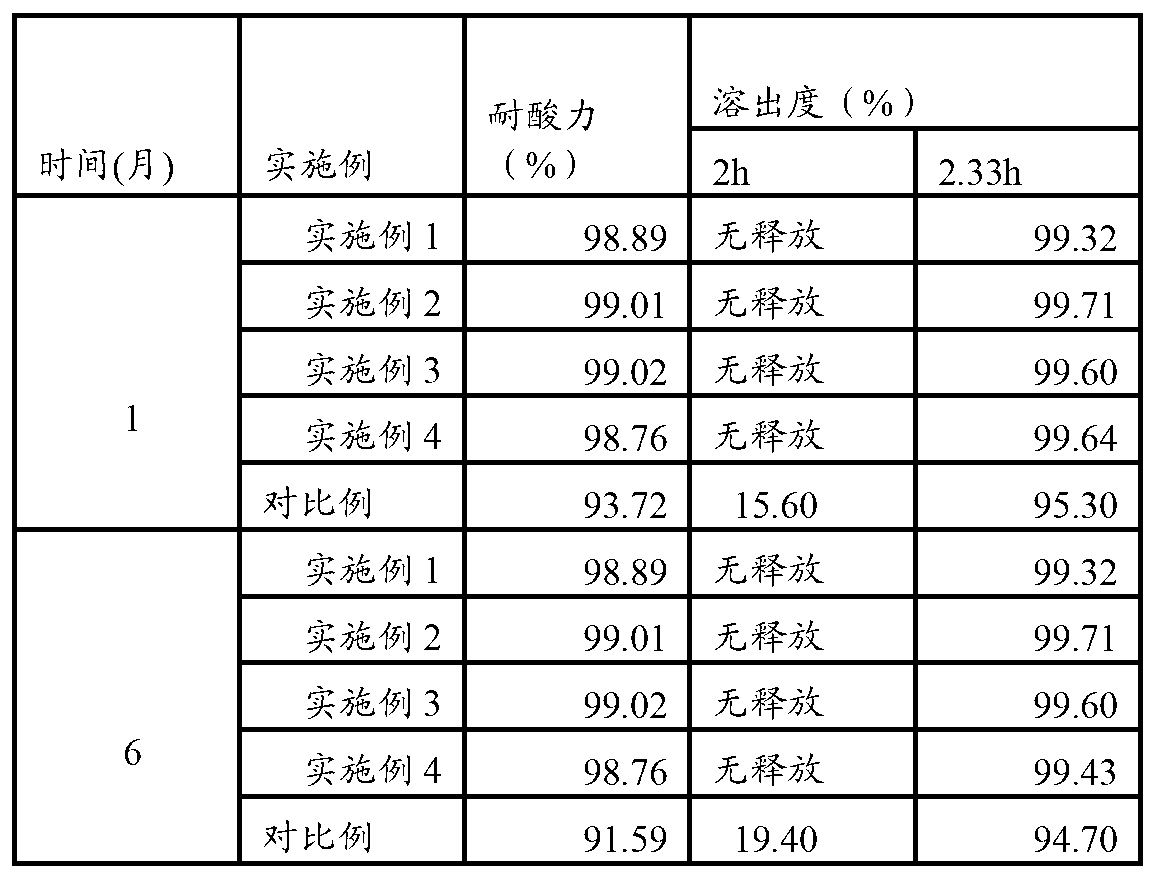
[0059] This embodiment provides an omeprazole enteric-coated capsule, comprising: a capsule shell and omeprazole pellets contained in the capsule shell, the particle size of the pellets is 15-30 mesh, and the omeprazole pellets From the inside to the outside, it is the core layer, the alkali layer, the isolation layer, the enteric layer and the outer layer. The mass ratio is 1:0.1:0.15:1.5:0.01, based on 10 parts by mass of omeprazole, Ogilvy & Mather The prescription of prazol pellets is shown in Table 1.
[0060] The above-mentioned preparation method of omeprazole enteric-coated capsules includes the following steps:
[0061] S1) Preparation of pellet core
[0062] Weigh omeprazole, lactose as a filler, hypromellose as a disintegrant, sodium carbonate as an alkaline sodium salt, and hypromellose as a binder according to the prescription in Table 1. A 120-mesh sieve is mixed to obtain a pellet core mixed powder.
[0063] Prepare the binder solution: Dissolve hypromellose in water ...
Embodiment 2
[0083] This embodiment provides an omeprazole enteric-coated capsule, comprising: a capsule shell and omeprazole pellets contained in the capsule shell, the particle size of the pellets is 15-30 mesh, and the omeprazole pellets From the inside to the outside, it is the core layer, the alkali layer, the isolation layer, the enteric layer and the outer layer. The mass ratio is 1:0.1:0.15:1.5:0.01, based on 10 parts by mass of omeprazole, Ogilvy & Mather The prescription of prazol pellets is shown in Table 1.
[0084] The above-mentioned preparation method of omeprazole enteric-coated capsules includes the following steps:
[0085] S1) Preparation of pellet core
[0086] According to the prescription in Table 1, weigh omeprazole, dextrin as a filler, sodium carboxymethyl starch as a disintegrant, sodium dihydrogen phosphate as an alkaline sodium salt, and povidone as a binder. A 120-mesh sieve is mixed to obtain a pellet core mixed powder.
[0087] Prepare the adhesive solution: Dissol...
Embodiment 3
[0103] This embodiment provides an omeprazole enteric-coated capsule, comprising: a capsule shell and omeprazole pellets contained in the capsule shell, the particle size of the pellets is 15-30 mesh, and the omeprazole pellets From the inside to the outside, it is the core layer, the alkali layer, the isolation layer, the enteric layer and the outer layer. The mass ratio is 1:0.1:0.15:1.5:0.01, based on 10 parts by mass of omeprazole, Ogilvy & Mather The prescription of prazol pellets is shown in Table 1.
[0104] The above-mentioned preparation method of omeprazole enteric-coated capsules includes the following steps:
[0105] S1) Preparation of pellet core
[0106] Weigh omeprazole, dibasic calcium phosphate as a filler, croscarmellose sodium as a disintegrant, disodium hydrogen phosphate as an alkaline sodium salt, and hydroxyl as a binder according to the prescription in Table 1. The propyl methyl cellulose is passed through a 120-mesh sieve and mixed to obtain a pellet core m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com